Alcohols
... ALCOHOLS • ALCOHOLS ARE A FAMILY OF ORGANIC COMPOUNDS THAT HAVE A HYDROXYL (-OH) GROUP ATTACHED TO A CARBON ATOM. • ALCOHOLS ARE VERY DIFFERENT FROM INORGANIC BASES. IN ALCOHOLS, THE HYDROXYL GROUP IS ATTACHED TO A CARBON BY A COVALENT BOND; THIS IS DIFFERENT FROM THE HYDROXIDE ION (OH-) WHICH IS A ...
... ALCOHOLS • ALCOHOLS ARE A FAMILY OF ORGANIC COMPOUNDS THAT HAVE A HYDROXYL (-OH) GROUP ATTACHED TO A CARBON ATOM. • ALCOHOLS ARE VERY DIFFERENT FROM INORGANIC BASES. IN ALCOHOLS, THE HYDROXYL GROUP IS ATTACHED TO A CARBON BY A COVALENT BOND; THIS IS DIFFERENT FROM THE HYDROXIDE ION (OH-) WHICH IS A ...
Fragmentations Associated with Organic Functional Groups
... It is known that fragmentation of n-alkyl ions occurs by loss of ethene (C2H4), so that the ion of m/z 57 in the mass spectrum of n-octane, above, is probably derived from two pathways, as shown below. ...
... It is known that fragmentation of n-alkyl ions occurs by loss of ethene (C2H4), so that the ion of m/z 57 in the mass spectrum of n-octane, above, is probably derived from two pathways, as shown below. ...
Chapter 4 – Carbon and the Molecular Diversity of Life
... responsible for the characteristic chemical reactions of those ...
... responsible for the characteristic chemical reactions of those ...
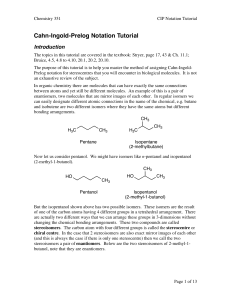
Cahn-Ingold-Prelog Notation Tutorial
... 1. The two highest priority groups are adjacent to each other in the Fischer projection and the lowest priority group is on a vertical bond. 2. The two highest priority groups are adjacent to each other in the Fischer projection and the lowest priority group is on a horizontal bond. 3. The two highe ...
... 1. The two highest priority groups are adjacent to each other in the Fischer projection and the lowest priority group is on a vertical bond. 2. The two highest priority groups are adjacent to each other in the Fischer projection and the lowest priority group is on a horizontal bond. 3. The two highe ...
Şenol, O.İ., Viljava, T.-R., Krause, AOI
... Nevertheless, the sulphided NiMo catalyst was more active than the sulphided CoMo catalyst. The conversion of both esters was complete at 275 8C on the sulphided NiMo catalyst and at 300 8C on the sulphided CoMo catalyst under the studied conditions. Comparison of the results with our earlier studie ...
... Nevertheless, the sulphided NiMo catalyst was more active than the sulphided CoMo catalyst. The conversion of both esters was complete at 275 8C on the sulphided NiMo catalyst and at 300 8C on the sulphided CoMo catalyst under the studied conditions. Comparison of the results with our earlier studie ...
5 Organic Chemistry
... The boiling point of alkanes increases as chain length increases. Explain this trend. n-pentane (CH3CH2CH2CH2CH3) is the straight chain isomer of pentane. (a) Draw the other 2 isomers (b) State the difference in boiling point between these isomers and n-pentane. (c) Explain this difference ...
... The boiling point of alkanes increases as chain length increases. Explain this trend. n-pentane (CH3CH2CH2CH2CH3) is the straight chain isomer of pentane. (a) Draw the other 2 isomers (b) State the difference in boiling point between these isomers and n-pentane. (c) Explain this difference ...
McMurray-Fay Chapter 22 Presentation Slides
... two or more different substituent groups are present, list them in alphabetical order. If two or more identical substituent groups are present, use one of the Greek prefixes: ...
... two or more different substituent groups are present, list them in alphabetical order. If two or more identical substituent groups are present, use one of the Greek prefixes: ...
Chemistry 30 – Organic Chemistry
... chain of carbon atoms. Name the long chain using its prefix with an ane ending. • Identify branches, and name using their prefix with a yl ending. ...
... chain of carbon atoms. Name the long chain using its prefix with an ane ending. • Identify branches, and name using their prefix with a yl ending. ...
Chapter 22 HEIN
... (2°), or tertiary (3°) depending on whether the carbon atom to which the –OH group is attached is directly bonded to one, two, or three other carbon atoms respectively. H C ...
... (2°), or tertiary (3°) depending on whether the carbon atom to which the –OH group is attached is directly bonded to one, two, or three other carbon atoms respectively. H C ...
Ch14 Lecture
... • Alcohols have two polar bonds, C—O and O—H, with a bent shape, therefore it has a net dipole. ...
... • Alcohols have two polar bonds, C—O and O—H, with a bent shape, therefore it has a net dipole. ...
Chapter 3
... octadecane, nondecane, eicosane (C20H42), heneicosane (C21H44), docosane (C22H46), tricosane (C23H48) ... tricontane (C30H62), ... tetracontane (C40H82) ... ...
... octadecane, nondecane, eicosane (C20H42), heneicosane (C21H44), docosane (C22H46), tricosane (C23H48) ... tricontane (C30H62), ... tetracontane (C40H82) ... ...
molecules
... temperature, filtered, washed with water and acetone and dried. The polymer supported porphyrin is insoluble in common organic solvents. The reflectance spectrum clearly indicates a Soret band at 488 nm and a Q band at 566 nm. IR spectrum of the solid supported manganese porphyrin shows (S=O) at 14 ...
... temperature, filtered, washed with water and acetone and dried. The polymer supported porphyrin is insoluble in common organic solvents. The reflectance spectrum clearly indicates a Soret band at 488 nm and a Q band at 566 nm. IR spectrum of the solid supported manganese porphyrin shows (S=O) at 14 ...
chapter 5: nomenclature
... What we have done is introduce a branch in the carbon chain. Now we must be able to distinguish between these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkan ...
... What we have done is introduce a branch in the carbon chain. Now we must be able to distinguish between these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkan ...
Chapter 14: Alcohols, Phenols, and Ethers This chapter is the first of
... Chapter 14: Alcohols, Phenols, and Ethers This chapter is the first of three that consider hydrocarbon derivatives with oxygen. This chapter is the first of three that consider hydrocarbon derivatives with o containing functional groups. Many biochemically important molecules contain carbon atoms bo ...
... Chapter 14: Alcohols, Phenols, and Ethers This chapter is the first of three that consider hydrocarbon derivatives with oxygen. This chapter is the first of three that consider hydrocarbon derivatives with o containing functional groups. Many biochemically important molecules contain carbon atoms bo ...
Hydrocarbons Note
... Using prefixes such as (n) or (iso) or (neo) might appear as a suitable method of nomenclature. This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of ...
... Using prefixes such as (n) or (iso) or (neo) might appear as a suitable method of nomenclature. This works for simple alkanes such as butane (C4H10) and pentane (C5H12). However, it becomes hopeless when larger alkanes are considered. For example there are 5 isomers of hexane (C6H14), 9 isomers of ...
Free Radical Chemistry and the Preparation of Alkyl
... Alkyl bromides, iodides or chlorides can react with Gilman or Grignard reagents in carbon-skeleton building substitution reactions (SN2 mechanism, Ch. 11) Alkyl halides and organic oxidation and reduction (10.9): Formation of Grignard or Gilman reagents can be considered an organic reduction since t ...
... Alkyl bromides, iodides or chlorides can react with Gilman or Grignard reagents in carbon-skeleton building substitution reactions (SN2 mechanism, Ch. 11) Alkyl halides and organic oxidation and reduction (10.9): Formation of Grignard or Gilman reagents can be considered an organic reduction since t ...
Chapter 20 Organic Chemistry
... The mirror image cannot be rotated so all its atoms align with the same atoms of the original molecule. ...
... The mirror image cannot be rotated so all its atoms align with the same atoms of the original molecule. ...
Ch22 Test
... 29. (Geometric, Optical) isomers result from different arrangements of four different groups about the same carbon atom. ____________________ 30. (Straight-chain, Branched-chain) alkanes contain carbon atoms that are bonded to more than two other carbon atoms. ____________________ 31. Organic compou ...
... 29. (Geometric, Optical) isomers result from different arrangements of four different groups about the same carbon atom. ____________________ 30. (Straight-chain, Branched-chain) alkanes contain carbon atoms that are bonded to more than two other carbon atoms. ____________________ 31. Organic compou ...
1 March 4, 2002 Exam 1 (Collaborative section) Organic Chemistry
... The alkylation of aromatic compounds is done using an alkyl halide and AlCl3. Depending on what is already on the ring, you may obtain one of several different products. The following reaction shows the three possible products one could obtain when nitro benzene is reacted with chloroethane. Circle ...
... The alkylation of aromatic compounds is done using an alkyl halide and AlCl3. Depending on what is already on the ring, you may obtain one of several different products. The following reaction shows the three possible products one could obtain when nitro benzene is reacted with chloroethane. Circle ...
full size
... group (methanal, the simplest, has two). ¾The group is somewhat more polar than ethers, but like ethers it cannot donate a hydrogen bond to itself. Thus aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less ...
... group (methanal, the simplest, has two). ¾The group is somewhat more polar than ethers, but like ethers it cannot donate a hydrogen bond to itself. Thus aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less ...
Document
... The molecules are polar if they have polar bonds, and if these bonds do not act in opposite directions and counteract each other. Polar molecules are attracted to each other by dipole-dipole forces. Polar molecules usually have a higher boiling point than similar non-polar molecules. Also, polar mol ...
... The molecules are polar if they have polar bonds, and if these bonds do not act in opposite directions and counteract each other. Polar molecules are attracted to each other by dipole-dipole forces. Polar molecules usually have a higher boiling point than similar non-polar molecules. Also, polar mol ...
Study Guide for Chapter 22 - Hydrocarbon Compounds
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
alcohols - A-Level Chemistry
... Identify the three isomers which can give two different alkenes when dehydrated and identify the possible alkene products in each case. Identify the one isomer which cannot undergo dehydration and explain why this is the ...
... Identify the three isomers which can give two different alkenes when dehydrated and identify the possible alkene products in each case. Identify the one isomer which cannot undergo dehydration and explain why this is the ...
Alkane
In organic chemistry, an alkane, or paraffin (a historical name that also has other meanings), is a saturated hydrocarbon. Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds. Alkanes (technically, always acyclic or open-chain compounds) have the general chemical formula CnH2n+2. For example, Methane is CH4, in which n=1 (n being the number of Carbon atoms). Alkanes belong to a homologous series of organic compounds in which the members differ by a molecular mass of 14.03u (mass of a methanediyl group, —CH2—, one carbon atom of mass 12.01u, and two hydrogen atoms of mass ≈1.01u each). There are two main commercial sources: petroleum (crude oil) and natural gas.Each carbon atom has 4 bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane e.g., C2-alkane.An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms, for example a methyl or ethyl group.The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydrocarbon. Waxes include examples of larger alkanes where the number of carbons in the carbon backbone is greater than about 17, above which the compounds are solids at standard ambient temperature and pressure (SATP).Alkanes are not very reactive and have little biological activity. All alkanes are colourless and odourless. Alkanes can be viewed as a molecular tree upon which can be hung the more biologically active/reactive portions (functional groups) of the molecule.