exam3 answers - Moorpark College
... A. List all the intermolecular forces that need to be overcome for the compound A given below to boil? ...
... A. List all the intermolecular forces that need to be overcome for the compound A given below to boil? ...
Double-Replacement Reactions - Fort Thomas Independent Schools
... • The classification scheme described in this section provides an introduction to five basic types of reactions: • Synthesis (Combination) • Decomposition • Single-displacement (replacement) • Double-displacement (replacement) • Combustion reactions ...
... • The classification scheme described in this section provides an introduction to five basic types of reactions: • Synthesis (Combination) • Decomposition • Single-displacement (replacement) • Double-displacement (replacement) • Combustion reactions ...
Section 2 Types of Chemical Reactions Chapter 8
... • The order in which the elements are listed is usually determined by single-displacement reactions. • The most-active element is placed at the top in the series. • It can replace each of the elements below it from a compound in a singledisplacement reaction. • Activity series are used to help predi ...
... • The order in which the elements are listed is usually determined by single-displacement reactions. • The most-active element is placed at the top in the series. • It can replace each of the elements below it from a compound in a singledisplacement reaction. • Activity series are used to help predi ...
a. b. c. d.
... acid (C6H5CH2COOH). Smell could help with the identity, but the worker has a cold, so samples of each of the three bottles are taken and H‐NMR, C‐NMR, and FTIR spectra of each bottle are made. Based on the spectra and the structures, which substance is in each bottle (A, B, and C). You must explai ...
... acid (C6H5CH2COOH). Smell could help with the identity, but the worker has a cold, so samples of each of the three bottles are taken and H‐NMR, C‐NMR, and FTIR spectra of each bottle are made. Based on the spectra and the structures, which substance is in each bottle (A, B, and C). You must explai ...
Organic Dyes as Photoredox Catalysts
... The Nicewicz group applied Mes–Acr+ as a photoredox catalyst for the intramolecular antiMarkovnikov hydroetherification and hydroamination of olefins to form cyclic ethers and amines, respectively.1 Intermolecular reactions with amines,2a carboxylic acids,1 and mineral acids2b are also possible unde ...
... The Nicewicz group applied Mes–Acr+ as a photoredox catalyst for the intramolecular antiMarkovnikov hydroetherification and hydroamination of olefins to form cyclic ethers and amines, respectively.1 Intermolecular reactions with amines,2a carboxylic acids,1 and mineral acids2b are also possible unde ...
Handout: Naming Organic Compounds Substituents Longest carbon
... alkyl groups attached to nitrogen as substituents. For same substituents, use “di” and “tri.” 2°, 3° amines with different R groups on N: Parent amine is the one with largest R group; name other groups as substituents, starting with N-. [Ions derived from amines: Replace –amine with –a ...
... alkyl groups attached to nitrogen as substituents. For same substituents, use “di” and “tri.” 2°, 3° amines with different R groups on N: Parent amine is the one with largest R group; name other groups as substituents, starting with N-. [Ions derived from amines: Replace –amine with –a ...
(the products). Mass is conserved in a chemical reaction
... • Given the reactants for a reaction, you can often predict the products that will form, as well as the relative amounts of reactants and products based on the patterns shown on the previous screen. • For example: ...
... • Given the reactants for a reaction, you can often predict the products that will form, as well as the relative amounts of reactants and products based on the patterns shown on the previous screen. • For example: ...
Name Page 1 F.05.215e3p1 I.
... related oxidizing agents, a 1,2-diol is formed. When phenylacetylene (Compound E) is treated with oxidizing agents such as OsO4, the corresponding addition reaction product is not observed; instead it is proposed that the initial addition reaction product, which is an ene-diol, undergoes an isomeriz ...
... related oxidizing agents, a 1,2-diol is formed. When phenylacetylene (Compound E) is treated with oxidizing agents such as OsO4, the corresponding addition reaction product is not observed; instead it is proposed that the initial addition reaction product, which is an ene-diol, undergoes an isomeriz ...
Synthesis of Oil of Wintergreen - Cornell University
... also with other carbon atoms. The ability of carbon to form chains, rings, and networks constitutes is responsible for the numerous number of organic compounds. Not only are carbon atoms linked by single covalent bonds, but they are sometimes linked by double or triple covalent bonds. Carbon atoms r ...
... also with other carbon atoms. The ability of carbon to form chains, rings, and networks constitutes is responsible for the numerous number of organic compounds. Not only are carbon atoms linked by single covalent bonds, but they are sometimes linked by double or triple covalent bonds. Carbon atoms r ...
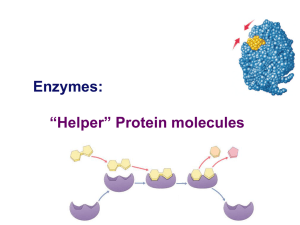
Enzymes: “Helper” Protein molecules
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
Mock Final Exam
... e. both A and C 6.5: Applying quantum mechanics to the atom 59. Define the term “orbital”. 60. What is required to move electrons from one orbital to another? 6.6: Orbital filling and electron configuration 61. What is the electron configuration of chlorine? 62. What is common to the electron config ...
... e. both A and C 6.5: Applying quantum mechanics to the atom 59. Define the term “orbital”. 60. What is required to move electrons from one orbital to another? 6.6: Orbital filling and electron configuration 61. What is the electron configuration of chlorine? 62. What is common to the electron config ...
types of reactions
... Most reactions fall into these five categories, and some may fall into more than one . ...
... Most reactions fall into these five categories, and some may fall into more than one . ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
Single Replacement Reactions
... with excess water and inform the instructor. Wear safety goggles and closed toed shoes throughout the entirety of the lab procedure.*** b. Label five test tubes - each with the name of one of the metals (zinc, aluminum, copper, iron and magnesium) if this has not been done already. c. Following your ...
... with excess water and inform the instructor. Wear safety goggles and closed toed shoes throughout the entirety of the lab procedure.*** b. Label five test tubes - each with the name of one of the metals (zinc, aluminum, copper, iron and magnesium) if this has not been done already. c. Following your ...
Organic Chemistry PowerPoint
... If there are more than 1 OH substituents the ending changes to 2-diol, 3-triol, 4-tetrol Common names have the alkyl group followed by “alcohol”. It can be a primary, secondary or tertiary alcohol due to the number of substituents attached to the carbon the OH is attached to. Practice problems #10 a ...
... If there are more than 1 OH substituents the ending changes to 2-diol, 3-triol, 4-tetrol Common names have the alkyl group followed by “alcohol”. It can be a primary, secondary or tertiary alcohol due to the number of substituents attached to the carbon the OH is attached to. Practice problems #10 a ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.