Organic Chemistry Review
... What is the functional group of a carboxylic acid? What is the ending given to carboxylic acid names? What is the name of these organics? ...
... What is the functional group of a carboxylic acid? What is the ending given to carboxylic acid names? What is the name of these organics? ...
M_ScOrganic_Chemistr..
... amino, carbonyl and carboxyl groups, synthetic strategies for cyclic compounds, retrosynthesis and synthetic approaches to some complex molecules such as camphor, longifoline, prostaglandins etc Unit 2 Carbon-carbon bond forming reactions: Knovenagel condensation, Darzon condensation. Michael additi ...
... amino, carbonyl and carboxyl groups, synthetic strategies for cyclic compounds, retrosynthesis and synthetic approaches to some complex molecules such as camphor, longifoline, prostaglandins etc Unit 2 Carbon-carbon bond forming reactions: Knovenagel condensation, Darzon condensation. Michael additi ...
isomeria geometrica
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
Carboxylic Acid Questions 1.-Arrange the following in order of (i
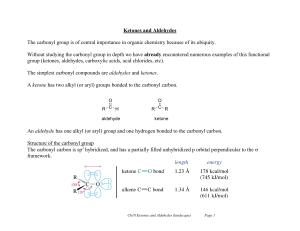
... Aldehydes are more reactive than ketones !!! as they are less hindered and the alkyl group in the ketone is a weak electron donor. Under the reaction condition s the carboxylic acid will deprotonate to give the carboxylate which is a very poor electrophile (after all, it has a negative charge !) so ...
... Aldehydes are more reactive than ketones !!! as they are less hindered and the alkyl group in the ketone is a weak electron donor. Under the reaction condition s the carboxylic acid will deprotonate to give the carboxylate which is a very poor electrophile (after all, it has a negative charge !) so ...
An Efficient Synthetic Route to Glycoamino Acid Building Blocks for
... During previous work on the synthesis of glycopeptides, two strategies have been employed. One approach introduces the carbohydrate as part of a glycoamino acid building block during solid-phase synthesis of the polypeptide chain.5 Alternatively, the carbohydrate can be attached to a selectively dep ...
... During previous work on the synthesis of glycopeptides, two strategies have been employed. One approach introduces the carbohydrate as part of a glycoamino acid building block during solid-phase synthesis of the polypeptide chain.5 Alternatively, the carbohydrate can be attached to a selectively dep ...
Chapter 10_Organohalides
... reaction pathway with Cl2 or Br2 and light (h) • Not very useful to the lack of control over the reaction and can lead ...
... reaction pathway with Cl2 or Br2 and light (h) • Not very useful to the lack of control over the reaction and can lead ...
Smith Reaction- HW PSI Chemistry
... 12) Chemical reactions _____.] A) occur only in living organisms B) create and destroy atoms C) only occur outside living organisms D) produce new substances 13) Which of the following is NOT a true statement concerning what happens in all chemical reactions? A) The ways in which atoms are joined t ...
... 12) Chemical reactions _____.] A) occur only in living organisms B) create and destroy atoms C) only occur outside living organisms D) produce new substances 13) Which of the following is NOT a true statement concerning what happens in all chemical reactions? A) The ways in which atoms are joined t ...
Organic Chemistry
... The rxn with KCN provides a means for extending the C-chain length by one C. The nitrile can then be converted either into amines by reduction using H2 with a Ni catalyst or into carboxyllic acid by acid hydrolysis. Example: ...
... The rxn with KCN provides a means for extending the C-chain length by one C. The nitrile can then be converted either into amines by reduction using H2 with a Ni catalyst or into carboxyllic acid by acid hydrolysis. Example: ...
chemistry and biological role of carbohydrates in the body-1
... Linkage is Defined as an: Acetal Linkage Between Carbonyl Carbon of a Monosaccharide and Hydoxyl Group of an Another Compound. ...
... Linkage is Defined as an: Acetal Linkage Between Carbonyl Carbon of a Monosaccharide and Hydoxyl Group of an Another Compound. ...
3.9alcohol rxns
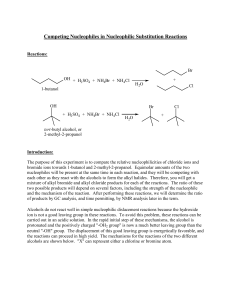
... Oxidation of Alcohols The reactions of alcohols have a central role in organic chemistry because alcohols can be converted to many of the other functional groups. Reduction is • a gain of electrons • a less positive oxidation number • a gain of hydrogen atoms • the loss of oxygen atoms • the loss o ...
... Oxidation of Alcohols The reactions of alcohols have a central role in organic chemistry because alcohols can be converted to many of the other functional groups. Reduction is • a gain of electrons • a less positive oxidation number • a gain of hydrogen atoms • the loss of oxygen atoms • the loss o ...
97KB - NZQA
... This reaction is a decomposition reaction, as a single reactant (hydrogen peroxide) forms two products (water and oxygen). Heat a small amount of each white solid in a boiling-tube. The boiling tube should have a bung in it, with a delivery tube going into a test-tube of limewater. If the limewater ...
... This reaction is a decomposition reaction, as a single reactant (hydrogen peroxide) forms two products (water and oxygen). Heat a small amount of each white solid in a boiling-tube. The boiling tube should have a bung in it, with a delivery tube going into a test-tube of limewater. If the limewater ...
3672 been studied in detail by Kebarle, et al., who
... Although little mechanistic work has been carried out on the nucleophilic cleavage of sulfenate esters,6 closely analogous reactions of trivalent phosphorus with both disulfides’ and peroxidess have been demonstrated to follow a mechanistically similar pathway. In direct analogy with these systems, ...
... Although little mechanistic work has been carried out on the nucleophilic cleavage of sulfenate esters,6 closely analogous reactions of trivalent phosphorus with both disulfides’ and peroxidess have been demonstrated to follow a mechanistically similar pathway. In direct analogy with these systems, ...
Chapter 7 Notes - Alcohols, Ethers, Thiols Functional groups alcohol
... CH3OH + NaNH2 ---> NH3 + CH3O- Na+ (sodium methoxide) most commonly made by direct reaction with active metals CH3OH + Na ---> 1/2 H2 + CH3O- Na+ (CH3)3COH + K ---> 1/2 H2 + (CH3)3CO-K+ ...
... CH3OH + NaNH2 ---> NH3 + CH3O- Na+ (sodium methoxide) most commonly made by direct reaction with active metals CH3OH + Na ---> 1/2 H2 + CH3O- Na+ (CH3)3COH + K ---> 1/2 H2 + (CH3)3CO-K+ ...
key to sample questions test 2
... h. Which of the following ions is most likely to be stable. (Hint: consider the Lewis structures) NH NH2 NH3 NH4 i. Which of the following molecules has a Lewis structure similar to that of N2? H2 O2 CO ...
... h. Which of the following ions is most likely to be stable. (Hint: consider the Lewis structures) NH NH2 NH3 NH4 i. Which of the following molecules has a Lewis structure similar to that of N2? H2 O2 CO ...
Chemical Reactions
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.