Document
... A5. An outline of the preparation of a halogenoalkane from an alcohol. Act A4.2 One way to make a halogenoalkane is to start with an alcohol and replace the – OH group by a halogen atom. Reaction in Activity A4.2: ...
... A5. An outline of the preparation of a halogenoalkane from an alcohol. Act A4.2 One way to make a halogenoalkane is to start with an alcohol and replace the – OH group by a halogen atom. Reaction in Activity A4.2: ...
Theoretical problems (official version)
... added much to our knowledge of this very complex process. One of such experiments was performed in 1930s by an English biochemist Robert Hill. In this problem, we consider some of his data together with the data of more recent experiments. 1. In plants, under illumination, carbon dioxide is reduced ...
... added much to our knowledge of this very complex process. One of such experiments was performed in 1930s by an English biochemist Robert Hill. In this problem, we consider some of his data together with the data of more recent experiments. 1. In plants, under illumination, carbon dioxide is reduced ...
WHAT IS MORPHINE -- ACTIVITY #1 What is morphine? What is it
... centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Secondary carbocation - Secondary carbocations have a pair of al ...
... centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Secondary carbocation - Secondary carbocations have a pair of al ...
File
... Show how MAO activates metal halide complexes containing bidentate ligands to act as polymerisation catalysts: use the general formula (L-L)MX2 to represent the pre-catalyst ...
... Show how MAO activates metal halide complexes containing bidentate ligands to act as polymerisation catalysts: use the general formula (L-L)MX2 to represent the pre-catalyst ...
Partial Periodic Table - Organic Chemistry at CU Boulder
... PLEASE read the questions carefully! ...
... PLEASE read the questions carefully! ...
Polarity
... difference in electronegativity between two bonded atoms • Not every polar bond results in a polar molecule • Polarity is largely determined by molecular geometry ...
... difference in electronegativity between two bonded atoms • Not every polar bond results in a polar molecule • Polarity is largely determined by molecular geometry ...
Transition Metal Carbonyls
... CO groups have a high tendency to stabilize M−M bonds; not only are CO ligands relatively small but they also leave the metal atom with a net charge similar to that in its elemental form (electroneutrality principle). “Stable complexes are those with structures such that each atom has only a small e ...
... CO groups have a high tendency to stabilize M−M bonds; not only are CO ligands relatively small but they also leave the metal atom with a net charge similar to that in its elemental form (electroneutrality principle). “Stable complexes are those with structures such that each atom has only a small e ...
Chem 174-Lecture 15a..
... and also changes the reactivity of the metal center based on X Variations of the cyclopentadiene moiety leads to the formation of catalyst that yield different forms of polypropylene (atatic, isotactic, syndiotactic) ...
... and also changes the reactivity of the metal center based on X Variations of the cyclopentadiene moiety leads to the formation of catalyst that yield different forms of polypropylene (atatic, isotactic, syndiotactic) ...
T10 AD bioenergetics
... mol/L (pH 0 !). How to convert reaction energetics to consider pH 7 rather than 0: 1. Use formula : DG= DGo + 5.69 kJ * log (P/S) DG= 49.53 kJ + 5.69 kJ * log (P/S) 2. Replace P by the concentration desired: DG= 49.53 kJ + 5.69 kJ * log (0.0000001 /S) = 49.53 kJ - 39.83 kJ = +9.7 kJ 3. Conclusion: R ...
... mol/L (pH 0 !). How to convert reaction energetics to consider pH 7 rather than 0: 1. Use formula : DG= DGo + 5.69 kJ * log (P/S) DG= 49.53 kJ + 5.69 kJ * log (P/S) 2. Replace P by the concentration desired: DG= 49.53 kJ + 5.69 kJ * log (0.0000001 /S) = 49.53 kJ - 39.83 kJ = +9.7 kJ 3. Conclusion: R ...
CBS Reduction
... • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol was produced with 95% yield and a 91 : 9 enantiomeric ratio (highly enantioselectivety). ...
... • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol was produced with 95% yield and a 91 : 9 enantiomeric ratio (highly enantioselectivety). ...
The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5
... base [9]. Kaoua and co-workers have reported the synthesis of diazepines by the reaction of ketimine intermediates and aldehydes in the presence of Keggin-Type heteropolyacids (HPA) [10]. The nucleophilic substitution of coumarincarbaldehyde derivatives with diamines resulted in the formation of 1,4 ...
... base [9]. Kaoua and co-workers have reported the synthesis of diazepines by the reaction of ketimine intermediates and aldehydes in the presence of Keggin-Type heteropolyacids (HPA) [10]. The nucleophilic substitution of coumarincarbaldehyde derivatives with diamines resulted in the formation of 1,4 ...
Carbon and Molecular Diversity
... has a L and D form. One is more active and found naturally, the other is frequent in vitamin pills but lacks the same biologic activity. • L-dopa example ...
... has a L and D form. One is more active and found naturally, the other is frequent in vitamin pills but lacks the same biologic activity. • L-dopa example ...
1.2 The Chemicals of Life - Father Michael McGivney
... • Stereoisomers that differ by having similar compounds on the same side or opposite sides of a rigid molecule (double bond, cyclic compound) • When single bonds exist between carbons, they can move, spin, rotate and flex to adjust their conformation. Carbons attached by double bonds or attached in ...
... • Stereoisomers that differ by having similar compounds on the same side or opposite sides of a rigid molecule (double bond, cyclic compound) • When single bonds exist between carbons, they can move, spin, rotate and flex to adjust their conformation. Carbons attached by double bonds or attached in ...
Chapter 17: Organic Chemistry
... • Expanded Structure shows each bond • Condensed Structures show each carbon atom and the attached hydrogen atoms ...
... • Expanded Structure shows each bond • Condensed Structures show each carbon atom and the attached hydrogen atoms ...
Empirical Formula
... • Oxygen gas (_____) is not explosive but must be present for combustion (fire) to occur • When these two gases come together to react, they form water – A molecule now with completely different properties!!! ...
... • Oxygen gas (_____) is not explosive but must be present for combustion (fire) to occur • When these two gases come together to react, they form water – A molecule now with completely different properties!!! ...
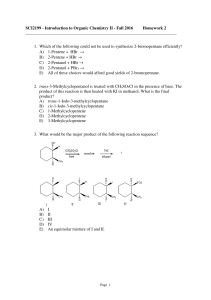
SCI2199 - Introduction to Organic Chemistry II
... A) trans-1-Iodo-3-methylcyclopentane B) cis-1-Iodo-3-methylcyclopentane C) 1-Methylcyclopentene D) 2-Methylcyclopentene E) 3-Methylcyclopentene 10. The reaction between 4-methyl-1-pentanol and PBr3 to yield 1-bromo-4methylpentane is probably: A) an SN1-type reaction involving the protonated alkyl di ...
... A) trans-1-Iodo-3-methylcyclopentane B) cis-1-Iodo-3-methylcyclopentane C) 1-Methylcyclopentene D) 2-Methylcyclopentene E) 3-Methylcyclopentene 10. The reaction between 4-methyl-1-pentanol and PBr3 to yield 1-bromo-4methylpentane is probably: A) an SN1-type reaction involving the protonated alkyl di ...
Chem 342 Jasperse Syllabus 1 Organic Chemistry II READING
... What’s Covered in Organic I versus Organic II differs between NDSU and MSUM The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end ...
... What’s Covered in Organic I versus Organic II differs between NDSU and MSUM The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end ...
ppt
... Carboxyl Has acidic properties because it is a source of hydrogen ions. The covalent bond between oxygen and hydrogen is so polar that hydrogen ions (H+) tend to dissociate reversibly; for example, ...
... Carboxyl Has acidic properties because it is a source of hydrogen ions. The covalent bond between oxygen and hydrogen is so polar that hydrogen ions (H+) tend to dissociate reversibly; for example, ...
Remodeling of the natural product fumagillol
... conditions was evaluated. When 11 was reacted with several anilines, the regioselectivity obtained with La(III) catalysis inverted, leading predominantly to perhydroisoquinoline products as originally found in the Zn(II)-catalyzed reactions (Figure 2b). By comparison, reaction of 11 using Zn(II) bec ...
... conditions was evaluated. When 11 was reacted with several anilines, the regioselectivity obtained with La(III) catalysis inverted, leading predominantly to perhydroisoquinoline products as originally found in the Zn(II)-catalyzed reactions (Figure 2b). By comparison, reaction of 11 using Zn(II) bec ...
Enzyme Activity
... Therefore, in the presence of a competitive inhibitor, more substrate is needed to achieve Vmax: Competitive inhibitors do not alter Vmax. The effect of a competitive inhibitor is reversed by increasing ...
... Therefore, in the presence of a competitive inhibitor, more substrate is needed to achieve Vmax: Competitive inhibitors do not alter Vmax. The effect of a competitive inhibitor is reversed by increasing ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.