* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download T10 AD bioenergetics
Survey
Document related concepts
Transcript
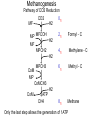
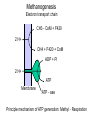
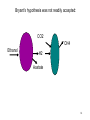
Bioenergetics of Anaerobic Microbial Reactions Traditional and new processes Methanogenesis Archaebacteria All methane producing bacteria (MPB) are archeabacteria Archeabacteria are different from all other life forms (3rd primary kingdom) Eukaryotes Plants Animals Fungi etc. This new taxonomic division of lifeforms into three kingdoms is based on phylogenetically conservative features: 1. Ether linked membrane lipids rather than ester links Archeabacteria Methanogens Halobacterium etc. Prokaryotes Eubacteria Cyanobacteria etc. 2. No murein in cell wall (→ penicillin resistant) 3. Different protein synthesis (→ streptomycin resistant) 4. More subunits in RNA polymerase 5. Unique coenzymes (e.g. F 420) Special features in archaebacteria: High temperature tolerance (115°C) Acid resistance (pH 1, at 90°C) Unique use of light ( no photosystem) Pyrodiction Sulfolobus Halobacterium Archaebacteria are likely to be the earliest life forms (Symbiont hypothesis) Methanogenesis Pathway of CO2 Reduction CO2 01 H2 MF MP MFCOH H2 MF MPCH2 H2 21 41 Methylene - C MPCH3 61 Methyl - C 81 Methane CoM MP CoMCH3 CoM ATP CH4 Formyl - C H2 Only the last step allows the generation of 1ATP Methanogenesis Electron transport chain CH3 - CoM + F420 2 H+ CH4 + F420 + CoM ADP + Pi 2 H+ ATP Membrane ATP - ase Principle mechanism of ATP generation: Methyl - Respiration Methanogenesis Acetate and Methanol Metabolism • During methanol conversion to methane only 1 ATP is generated via methyl – respiration. 4 CH3OH → 3 CH4 + CO2 + 2 H2O 61 81 01 • In the presence of hydrogen methanol is only used as electron acceptor → methyl – respiration. H2 + CH3OH → CH4 + H2O 20 61 81 • Acetoclastic methanogens split acetate into a methyl group and a carboxy group. The two electrons of the carboxy group are transferred to the methyl group → methyl – respiration. CH3 - COOH → CH4 + CO2 61 21 81 01 82 Methyl - respiration is the only way of ATP generation in methanogenesis Overall the energy conserving reaction in methanogenesis is from proton translocation via electron transfer to the methyl group generated during metabolism. Methanogenesis = anaerobic methyl respiration Revision: Gibbs Free Energy Change The Gibbs Free Energy (G) of substrates and products can be calculated. For exergonic (=spontaneous = downhill) reactions the G of the substrates is higher than that of the products. Hence the change in G (=ΔG) for exergonic reactions is negative. This ΔG is the driving force of the reaction. As the reaction proceeds, the diminishing substrate concentration and increasing product concentration cause the difference in G to become smaller and smaller until an equilibrium is reached at which ΔG = zero. Revision: Gibbs Free Energy Change Handbooks provide values of the Gibbs Free Energy Change as: ΔGo assumes that all reactants and products are a unity (concentrations are 1 M and gas partial pressures are 100 kPa) ΔGo’ assumes that all reactants and products are at unity, however the proton concentration is not 1 M (pH 0) but 10-7 M ( pH 7). This value makes more sense for most biological systems ΔG is the actual Free Energy Change under experimental conditions and changes any moment as the reaction proceeds Methanobacillus omelianskii, observed features 1. degrades ethanol to acetate and methane gas: CH3-CH2OH + CO2 --> CH3-COOH + CH4 2. Also grows with H2 as e-donor and CO2 as e- acceptor: 4H2 + CO2 --> CH4 + 2H2O Suspicions about the purity of culture were raised because: 1. H2 inhibited ethanol degradation but not CH4 production (Preference for H2?) 2. No growth on ethanol after prolonged cultivation on H2 + CO2 (Mutagenic loss of ethanol dehydrogenease?) 3. Bubbling inert N2 gas through ethanol degrading culture --> CH4 production stopped but ethanol oxidation to acetate continued. 11 Theory of Bryant on Interspecies Hydrogen Transfer Hypothesis of Marvin P. Bryant: Interspecies Hydrogen Transfer, CO2 CH4 Ethanol H2 Acetate Claims: 1. Methanobacillus consists of an association of two microbes: 2. Pelobacter depends on H2 removal by MPB or N2 flushing. 3. MPB needs needs Pelobacter to provide H2 as e-donor. 4. Both depend on each other (syntrophy) 12 Bryant’s hypothesis was not readily accepted: Lack of driving force of reaction 1: CH3-CH2OH + H2O --> CH3-COO- + H+ + 2 H2 DGo'= + 9.6 kJ/mol CO2 CH4 Ethanol H2 Acetate Bryant's argument: reaction 2 has excess driving force 4 H2 + HCO3- + H+ --> CH4 + 3 H2O DGo'= - 135.6 kJ/mol Total reaction: DGo'= - 116.4 kJ/mol Question: Energy sharing possible (pulley analogy)? 13 Bryant’s hypothesis was not readily accepted: CO2 CH4 Ethanol H2 Acetate 14 Energy Sharing DGo'= + 9.6 kJ/mol How can two different microbes share the common energetic potential? The actual free energy change of the reaction DG depends on the product to substrate ratio (P/S) and can be calculated from the standard DG= DGo + 5.69 kJ * log (P/S) DGo'= - 135.6 kJ/mol CH4 CO2 Ethanol H2 Acetate For high substrate concentrations (P/S less than 1) the DG will be more favourable (negative). Interspecies hydrogen transfer was postulated to operate at extremely low H2 partial pressures o 1- 10 Pa (compared to 100,000 Pa for standard conditions) 15 Energy Sharing DGo'= + 9.6 kJ/mol Interspecies hydrogen transfer was postulated to operate at extremely low H2 partial pressures o 1- 10 Pa (compared to 100,000 Pa for standard conditions. Low H2 increases the driving force of reaction 1 while it decreases the driving force of reaction 2. DGo'= - 135.6 kJ/mol CH4 CO2 Ethanol H2 Acetate Reaction1 Reaction 2 A sharing in driving force is possible by lowering H2 until reaction 1 becomes feasible (exergonic) but not so low that reaction 2 becomes endergonic. 16 Significance of Interspecies Hydrogen Transfer Eventually Bryan’s hypothesis was not only confirmed but found to be a general feature of anaerobic systems (e.g. rumen, anaerobic digesters, sediments) DGo'= + 9.6 kJ/mol DGo'= - 135.6 kJ/mol CH4 CO2 Ethanol H2 Acetate The interspecies hydrogn can be intercepted by chemical and biological means (more powerful H2 users than methanogens. Reaction1 Reaction 2 Other substrates found to degrade via H2 transfer include all fatty acids, alcohols, many amino acids 30 % of electron flow in anaerobic digesters passes via H2. Use of energetic calculations critical to understand (exploit, control, optimise) many biological reactions) 17 How can the energetic values be determined ?: The example of Methanobacillus omelianskii and interspecies hydrogen transfer has shown the importance of DG calculations: Example problem: Establish the standard DGo for the anaerobic conversion of ethanol (12 e-) to acetate (8 e-) by Pelobacter (syntrophic partner in M. omlianskii) 1. Establish proper equation: (4 electrons transferred) CH3-CH2OH + H2O <--> CH3-COO- + H+ + 2 H2 (-181.75) + (-237.18) <--> (-369.4) + 0 + 0 2. Look up the standard Gibbs free energy of formation (Gfo) 3. Subtract the Gfo of substrates from Gfo of products --> DGo= + 49.53 kJ/mol 4. This gives the energetic situation (negative = spontaneous = exergonic = downhill) for standard conditions: Room temperature, partial pressure of all gases = 100 kPa, all concentrations 1 mol/L. Why is the established value not the one calculated by Bryant for the 18 same reaction? Considering pH for thermodynamic calculations DG= DGo + 5.69 kJ * log (P/S) Standard conditions imply that also protons are at a concentration of 1 mol/L (pH 0 !). How to convert reaction energetics to consider pH 7 rather than 0: 1. Use formula : DG= DGo + 5.69 kJ * log (P/S) DG= 49.53 kJ + 5.69 kJ * log (P/S) 2. Replace P by the concentration desired: DG= 49.53 kJ + 5.69 kJ * log (0.0000001 /S) = 49.53 kJ - 39.83 kJ = +9.7 kJ 3. Conclusion: Reaction requires less energy to be run but is still not spontaneous as DG is positive. What about the other products and substrates ? 19 How to calculate actual reaction energetics by considering all P and S concentrations CH3-CH2OH + H2O --> CH3-COO- + H+ + 2 H2 DG= DGo + 5.69 kJ * log ((P1*P2*P3 ) / (S1*S2*S3)) Example : Does the reaction become favourable given that P1= aceate =1 mM, = 0.001 of std. cond. P2= protons = 10-7 M P3=H2 = 10 Pa = 0.0001 of std. cond. S1= ethanol = 10 mM = 0.01 of std. cond. 1. Use formula : DG= 49.53 kJ + 5.69 kJ * log (P1*P2*P3/(S1*S2*S3)) 2. Replace P and S by the concentrations desired: DG= 49.53 kJ + 5.69 kJ * log (0.001*10-7*(0.0001)2/(0.01*1))= DG= 49.53 kJ - 5.69 kJ * log 10-16= 49.53 kJ - 5.69 kJ * 16 = -41.51 kJ 3. Conclusion: Reaction is now thermodynamically possible and can support growth. 20 Visualising Effect of Substrate and Product concentration on Reaction Energetics Page 12:46 in study guide The G calculations of the previous slides conclude that: 1. [substrate] energy released (more downhill) 2. [product] energy released (less downhill) +100 +100 G 0 G 0 0.1 1 10 100 -100 Effect of substrate concentration on energetics of the reaction 0.1 1 10 100 -100 Effect of product concentration on energetics of reaction Remember: energy released means the change in energy is negative. energy released means G becomes more negative 21 Visualising Effect of H2 on energetics of H2 consumption and Production Page 12:46 in study guide The G calculations of the previous slides conclude that: 1. [H2] energy released for H2 consumption 2. [H2] energy released for H2 production +100 +100 G 0 G 0 0.1 1 10 -100 Effect of H2 on energetics of H2 consumption 100 0.1 1 10 100 -100 Effect of H2 on energetics of H2 production Remember: energy released means the change in energy is negative. energy released means G becomes more negative 22 Visualising Effect of H2 on energetics of H2 consumption and Production Page 12:46 in study guide The G calculations of the previous slides conclude that: 1. [H2] energy released for H2 consumption 2. [H2] energy released for H2 production There is only a narrow concentration range at which both reactions are energetically favourable (G negative). +100 G 0 0.1 1 10 100 -100 Effect of H2 on energetics of H2 consumption and production Both reactions can co-exist. Both bacterial groups can obtain energy. Lowering H2 by one microbe improves the energetic situation of the other (energy sharing). Visualising Effect of H2 on energetics of H2 consumption and Production Page 12:46 in study guide 1,000,000 100,000 In text books [H2] is often plotted against G. 10,000 [H2] (ppm) 10 G positive endergonic “uphill” G=0 G negative exergonic “downhill” 24 Anaerobic digestion, a 3 stage process Complex organic matter (starch, cellulose, fats, protein) In contrast to aerobic degradation, AD requires different groups of specialised bacteria: Fermentative Bacteria (e.g. Clostridia) Hydrolysis and fermentation Volatile fatty acids (VFA) alcohols, amino acids Acetogenesis via hydrogen production OHPA H2 CO2 Methanogenesis of acetate and H2 Acetate MPB CO2 CH4 25 Anaerobic digestion, role of H2 concentration 1/3 of the electron flow proceeds via H2 H2 concentration is very low (1/ 40,000 of atmosphere = 40 ppm) but flux is high If H2 increases higher than 100 ppm to 1000 ppm the OHPA can not operate any more (DG is positive) Extremely low H2 level is critical Digester overloading with easily fermetable organics (sugars) fast H2 production H2 production > H2 consumption H2 accumulation OHPA don’t convert organic acids fermenting bacteria produce more VFA than H2 acetate drop in pH killing MPB Overloading of anaerobic digesters with excess substrate results in failure due to H2 buildup Bottleneck similar to Crabtree effect , acidification 26 What is the expected CH4 concentration in biogas for different organics Compound Glucose Ethanol Lactate Propionate Butyrate Butanol Oxalate Oxalate Formate Methanol Methane CO2 Electons Carbons 246 122 123 143 204 246 22 22 21 61 81 01 E/C OS %CH4 content 4 6 4 4.7 5 6 1 1 2 6 8 0 0 -2 0 -0.7 -1 -2 3 3 2 -2 -4 +4 50 75 50 59 62.5 75 12.5 12.5 25 75 100 0 27