aryl halides
... sp2 hybridized and not prone to nucleophilic substitution. In a manner analogous to the phenols & alcohols, we have the same functional group in the two families, aryl halides and alkyl halides, but very different chemistries. ...
... sp2 hybridized and not prone to nucleophilic substitution. In a manner analogous to the phenols & alcohols, we have the same functional group in the two families, aryl halides and alkyl halides, but very different chemistries. ...
SAMPLE QUESTION PAPER SIR.S.M.TAHIR CHEMISTRY Mob: 9557076999
... Explain, why does the atomic radii increases considerably from N to P but very little increase is observed from As to Bi. ...
... Explain, why does the atomic radii increases considerably from N to P but very little increase is observed from As to Bi. ...
M.Sc. II - Punjabi University
... formation of complexes from aqueous ions, equation and base hydrolysis, attack on ligands. (c) Reactions of Square complexes- mechanism of ligand displacement reactions, the trans effect, cis-effect. SECTION-B 16 Hrs. Metal Carbonyl reactions- Reactions of octahedral, reactions of binuclear carbonyl ...
... formation of complexes from aqueous ions, equation and base hydrolysis, attack on ligands. (c) Reactions of Square complexes- mechanism of ligand displacement reactions, the trans effect, cis-effect. SECTION-B 16 Hrs. Metal Carbonyl reactions- Reactions of octahedral, reactions of binuclear carbonyl ...
Organic Chemistry II
... Cyclohexanol is a secondary alcohol, which can be protonated by a strong acid. The protonated alcohol then loses a water molecule to form a secondary carbocation. Loss of a hydrogen atom on an adjacent carbon atom results in the formation of cyclohexene. It is possible that a substitution reaction c ...
... Cyclohexanol is a secondary alcohol, which can be protonated by a strong acid. The protonated alcohol then loses a water molecule to form a secondary carbocation. Loss of a hydrogen atom on an adjacent carbon atom results in the formation of cyclohexene. It is possible that a substitution reaction c ...
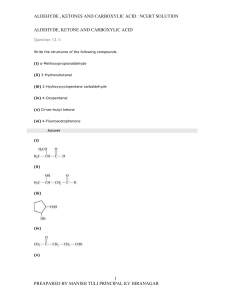
carbonyl compound group
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
Chapter 15: Kinetics
... mol/L s at the start of the reaction what are the rates of change for B, C and D at this time? Rate of change of B = Rate of change of C = Rate of change of D = a) B= 0.042 M/s; C= 0.056 M/s; D= - 0.042 mol/L s b) B = -0.042M/s; C = 0.112 M/s; D = 0.042 mol/L s c) B= -0.042 M/s; C= - 0.112 M/s; D= 0 ...
... mol/L s at the start of the reaction what are the rates of change for B, C and D at this time? Rate of change of B = Rate of change of C = Rate of change of D = a) B= 0.042 M/s; C= 0.056 M/s; D= - 0.042 mol/L s b) B = -0.042M/s; C = 0.112 M/s; D = 0.042 mol/L s c) B= -0.042 M/s; C= - 0.112 M/s; D= 0 ...
KEY + + - UIC Department of Chemistry
... of product which you believe to be N2 . Is that possible? Explain your answer. (7 points) mass N2 possible = (0.0880778 mol NH3)(2 mol N2/4 mol NH3)(28.0134 g/1 mol N2) = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
... of product which you believe to be N2 . Is that possible? Explain your answer. (7 points) mass N2 possible = (0.0880778 mol NH3)(2 mol N2/4 mol NH3)(28.0134 g/1 mol N2) = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
chapter 8
... equations. As you can see, some things can be shown in different ways. For example, sometimes a gaseous product is indicated by an arrow pointing upward,↑, instead of (g). A downward arrow, ↓, is often used to show the formation of a precipitate during a reaction in solution. The conditions under wh ...
... equations. As you can see, some things can be shown in different ways. For example, sometimes a gaseous product is indicated by an arrow pointing upward,↑, instead of (g). A downward arrow, ↓, is often used to show the formation of a precipitate during a reaction in solution. The conditions under wh ...
Alcohols, Phenols and Ethers
... water. The reaction with R’ COCI is carried out in the presence of pyridine so as to neutralise HCI which is formed during the reaction. The introduction of acetyl (CH3CO-) group in phenols is known as acetylation. Acetylation of salicylic acid produces aspirin. ...
... water. The reaction with R’ COCI is carried out in the presence of pyridine so as to neutralise HCI which is formed during the reaction. The introduction of acetyl (CH3CO-) group in phenols is known as acetylation. Acetylation of salicylic acid produces aspirin. ...
the beginnings of synthetic organic chemistry: zinc alkyls and the
... organophosphorus chemistry. The greater ease of formation and use of the Grignard reagent made it generally superior to the corresponding zinc reagent, especially in the hands of less experienced chemists. The very dominance of the Grignard reagent after 1900, however, pays silent testimony to the e ...
... organophosphorus chemistry. The greater ease of formation and use of the Grignard reagent made it generally superior to the corresponding zinc reagent, especially in the hands of less experienced chemists. The very dominance of the Grignard reagent after 1900, however, pays silent testimony to the e ...
towards the synthesis of functionalised macrocyclic receptors
... The Williamson ether synthesis is commonly used in the synthesis of macrocyclic polyethers. This classic reaction involves the SN2 displacement of an alkyl halide with an alkoxide anion21 when a diol and dihalide are reacted together in presence of a base. Recently it was reported that the halide le ...
... The Williamson ether synthesis is commonly used in the synthesis of macrocyclic polyethers. This classic reaction involves the SN2 displacement of an alkyl halide with an alkoxide anion21 when a diol and dihalide are reacted together in presence of a base. Recently it was reported that the halide le ...
Part 2 ir spectra_01
... Alcohols and amines display strong broad O-H and NH stretching bands in the region 3400-3100 cm-1. The bands are broadened due to hydrogen bonding and a sharp 'non-bonded' peak can often be seen at around 3400 cm-1. Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100- ...
... Alcohols and amines display strong broad O-H and NH stretching bands in the region 3400-3100 cm-1. The bands are broadened due to hydrogen bonding and a sharp 'non-bonded' peak can often be seen at around 3400 cm-1. Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100- ...
Document
... Pressure and Volume Reaction involves no change in the number moles of gas ◦ No effect on composition of equilibrium mixture For heterogenous equilibrium mixture ◦ Effect of pressure changes on solids and liquids can be ignored Volume is nearly independent of pressure Change in pressure due to ...
... Pressure and Volume Reaction involves no change in the number moles of gas ◦ No effect on composition of equilibrium mixture For heterogenous equilibrium mixture ◦ Effect of pressure changes on solids and liquids can be ignored Volume is nearly independent of pressure Change in pressure due to ...
EASTERN ARIZONA COLLEGE General Organic Chemistry I
... Predict which substitutions or eliminations will be faster, based on differences in substrate, base/nucleophile, leaving group, or solvent. ...
... Predict which substitutions or eliminations will be faster, based on differences in substrate, base/nucleophile, leaving group, or solvent. ...
fulltext
... findings. An enzyme is able to bind one or more distinct molecules or atoms, its substrates, and provide an environment which is more favourable for a chemical reaction of the substrate(s) than the surrounding solution. The location in the enzyme where this takes place is called the active site. Acc ...
... findings. An enzyme is able to bind one or more distinct molecules or atoms, its substrates, and provide an environment which is more favourable for a chemical reaction of the substrate(s) than the surrounding solution. The location in the enzyme where this takes place is called the active site. Acc ...
CHEMISTRY 263
... Do Not turn in, answers available in "Study Guide and Solutions Manual for Organic Chemistry" for Solomons. This is available in the Bookstore or can be borrowed from Cameron Library's Reserve Reading Room Chapter 11: 11.2 to11.4; 11.9; 11.13 to 11.16; 11.25 to 11.27; 11.34 Chapter 22: 22.1; 22. ...
... Do Not turn in, answers available in "Study Guide and Solutions Manual for Organic Chemistry" for Solomons. This is available in the Bookstore or can be borrowed from Cameron Library's Reserve Reading Room Chapter 11: 11.2 to11.4; 11.9; 11.13 to 11.16; 11.25 to 11.27; 11.34 Chapter 22: 22.1; 22. ...
Ch 15 - Phillips Scientific Methods
... • Chymotrypsin has 251 stereocenters. • The maximum number of stereoisomers possible is ...
... • Chymotrypsin has 251 stereocenters. • The maximum number of stereoisomers possible is ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.