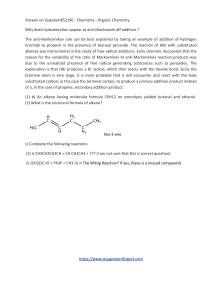
Honors Chemistry Organic Chemistry
... 3. Which term doesn’t go with the others? (Circle one) alkyl group substituent division ...
... 3. Which term doesn’t go with the others? (Circle one) alkyl group substituent division ...
Exam 2 Review A
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
... three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussion of #3 until Chapter 7]. Remember, carbocation stability plays a role in analyzing transition states, whic ...
Aldehydes and Ketones
... How can we tell if we’ll be getting carbonyl addition or conjugate addition? Fortunately, it is quite easy. Non-carbon nucleophiles (we frequently call these “soft” nucleophiles) like nitrogen (amines) and oxygen (alcohols) will always add in a conjugate fashion, particularly if the reaction is carr ...
... How can we tell if we’ll be getting carbonyl addition or conjugate addition? Fortunately, it is quite easy. Non-carbon nucleophiles (we frequently call these “soft” nucleophiles) like nitrogen (amines) and oxygen (alcohols) will always add in a conjugate fashion, particularly if the reaction is carr ...
15 - MSU Chemistry
... The starting material in the first reaction has a plane of symmetry so it is achiral: the stereochemistry shows only which diastereoisomer we have. Attack by the amine nucleophile at either end ...
... The starting material in the first reaction has a plane of symmetry so it is achiral: the stereochemistry shows only which diastereoisomer we have. Attack by the amine nucleophile at either end ...
Organic Tutorial 1st Year HT01
... 2. "Core Carbonyl Chemistry", John Jones, OUP Primer No 47. 3. "Mechanisms of organic reactions", H.Maskill, OUP Primer No 45. Introduction Nucleophilic attack at carbonyl compounds was covered in a previous tutorial. This tutorial aims to cover another major function of carbonyl compounds: enolisat ...
... 2. "Core Carbonyl Chemistry", John Jones, OUP Primer No 47. 3. "Mechanisms of organic reactions", H.Maskill, OUP Primer No 45. Introduction Nucleophilic attack at carbonyl compounds was covered in a previous tutorial. This tutorial aims to cover another major function of carbonyl compounds: enolisat ...
Ch 12 Alcohols and Thiols
... 2. Van der Waals – depending on Carbon chain length • Length of Carbon chain • C1-C2 gas, C3 -C10: liquid, C11 and higher: solids ...
... 2. Van der Waals – depending on Carbon chain length • Length of Carbon chain • C1-C2 gas, C3 -C10: liquid, C11 and higher: solids ...
Lecture #
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
Microsoft Word - Final Exam Study Guide
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
- professional publication
... Reaction. Mechanism and Stereochemistry of S N2 reaction, Mechanism and Stereochemistry of SN1 Reaction. Rearrangement of Carbocation, S N2 versus SN1 Reactions, Reactivity of Alkyl Halides in SN1 and SN2, Factors Affecting SN1 and SN2. ...
... Reaction. Mechanism and Stereochemistry of S N2 reaction, Mechanism and Stereochemistry of SN1 Reaction. Rearrangement of Carbocation, S N2 versus SN1 Reactions, Reactivity of Alkyl Halides in SN1 and SN2, Factors Affecting SN1 and SN2. ...
Preparation of alkyl halides There are lots of ways to make alkyl
... to use one of the special halogenating agents shown below: ...
... to use one of the special halogenating agents shown below: ...
Study_guide_2010-01
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
TV RajanBabu Chemistry, 730 Autumn 1997
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
Chem 263 Nov 3 2016 notes
... nucleophile to form the alkoxide anion, which is then protonated. Most such reactions are done under acidic conditions, in which case the mechanism involves initial protonation of the oxygen. Under basic conditions, many such reactions have an equilibrium that lies to the side of starting materials ...
... nucleophile to form the alkoxide anion, which is then protonated. Most such reactions are done under acidic conditions, in which case the mechanism involves initial protonation of the oxygen. Under basic conditions, many such reactions have an equilibrium that lies to the side of starting materials ...
T. V. RajanBabu Chemistry, 730 Autumn 1997
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
730-2005 topics
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
Redox Reactions
... Hydride reagents provide H- as a nucleophile, therefore they only react with polar substrates containing an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the ca ...
... Hydride reagents provide H- as a nucleophile, therefore they only react with polar substrates containing an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the ca ...
Study guide/lecture topics
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
... Chemistry 335 List of topics/study guide. This is a list of topics we will be covering to help you in preparation for exams. Topics from Clayden are indicated clearly by chapter and page numbers where necessary. Topics NOT from Clayden are listed in italics. PLTL topics are in CAPS. This document wi ...
Chapter 11: Reactions at an sp3 Hybridized Carbon III
... • In this case, however, the stability of tertiary carbocation which results from H– shifting and substituting for CH3OH makes this reaction work with HCl • If tertiary carbocations can be formed then HCl is strong enough to cleave ethers ...
... • In this case, however, the stability of tertiary carbocation which results from H– shifting and substituting for CH3OH makes this reaction work with HCl • If tertiary carbocations can be formed then HCl is strong enough to cleave ethers ...
The carbonyl group
... • The carbonyl group (C=O) is found in aldehydes, ketones, and many other organic functional groups. ...
... • The carbonyl group (C=O) is found in aldehydes, ketones, and many other organic functional groups. ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... clear separation had occurred, leading to rotary vaporization of the separate substances. However, characterization of the separation yielded inconclusive spectra. Thus, the BOC deprotection step was proceeded on the product from trial two. Deprotection yielded spectra that plausibly matched chemica ...
... clear separation had occurred, leading to rotary vaporization of the separate substances. However, characterization of the separation yielded inconclusive spectra. Thus, the BOC deprotection step was proceeded on the product from trial two. Deprotection yielded spectra that plausibly matched chemica ...
... to chiral alcohols has attracted much attention, since many chiral alcohols are highly valuable intermediates for preparing chiral pharmaceutical and agricultural products. Despite the organoaluminium reagents are economically obtained in industrial scale, their use is rare. In this respect, the few ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.