CHM230 OXIDATION OF CYCLOHEXANOL TO CYCLOHEXANONE
... graduated cylinder. The distillate should be a mixture of cyclohexanone and water that contains excess acetic acid. RECORD the boiling point of the cyclohexanone. Transfer the distillate to a separatory funnel or beaker. 6. Add 3.5 grams of sodium carbonate to neutralize any excess acetic acid, and ...
... graduated cylinder. The distillate should be a mixture of cyclohexanone and water that contains excess acetic acid. RECORD the boiling point of the cyclohexanone. Transfer the distillate to a separatory funnel or beaker. 6. Add 3.5 grams of sodium carbonate to neutralize any excess acetic acid, and ...
top 5 organic - No Brain Too Small
... Preparation method (alc + c.acid); heat under reflux; add carbonate to neutralise acids, separate ester from impurities by distillation Breaking – reaction with H2O - hydrolysis (break where you make – i.e between O and C=O) ...
... Preparation method (alc + c.acid); heat under reflux; add carbonate to neutralise acids, separate ester from impurities by distillation Breaking – reaction with H2O - hydrolysis (break where you make – i.e between O and C=O) ...
Ch 26 C-C bond formation
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
Organic Reactions
... Formation of a polypeptide • Polypeptide is a chain of amino acids, each amino acid has one carboxylic acid and one amine group • Note that the polymerization here occurs because there are two different groups on the same molecule • Polypeptides are not, technically, polymers since they don’t have ...
... Formation of a polypeptide • Polypeptide is a chain of amino acids, each amino acid has one carboxylic acid and one amine group • Note that the polymerization here occurs because there are two different groups on the same molecule • Polypeptides are not, technically, polymers since they don’t have ...
Lecture Resource ()
... The reactivity of carbonyl compounds resides in the polarity of the carbonyl group in a nucleophilic acyl substitution reaction ...
... The reactivity of carbonyl compounds resides in the polarity of the carbonyl group in a nucleophilic acyl substitution reaction ...
PPT file
... The mechanism for this reaction is very similar to that for addition of water to a carbonyl group; however, because amines are better nucleophiles than alcohols/water, the carbonyl group doesn’t have to be protonated first. A small amount of acid is necessary to generate a leaving group of water (R- ...
... The mechanism for this reaction is very similar to that for addition of water to a carbonyl group; however, because amines are better nucleophiles than alcohols/water, the carbonyl group doesn’t have to be protonated first. A small amount of acid is necessary to generate a leaving group of water (R- ...
Reaction of Organometallic Reagents with Aldehydes and Ketones.
... • The reaction follows the same two-step process as opening of epoxide rings with other negatively charged nucleophiles—that is, nucleophilic attack from the back side of the epoxide, followed by protonation of the resulting alkoxide. • In unsymmetrical epoxides, nucleophilic attack occurs at the le ...
... • The reaction follows the same two-step process as opening of epoxide rings with other negatively charged nucleophiles—that is, nucleophilic attack from the back side of the epoxide, followed by protonation of the resulting alkoxide. • In unsymmetrical epoxides, nucleophilic attack occurs at the le ...
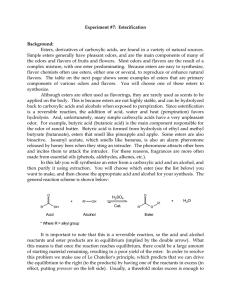
Lab 7_Esterification
... of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to the products) by having one of the reactants in excess (in effect, putting pressure on t ...
... of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to the products) by having one of the reactants in excess (in effect, putting pressure on t ...
ketones - Fisanti Site
... primary alcohols. • Organometallics with carbon dioxide yield carboxylic acids. ...
... primary alcohols. • Organometallics with carbon dioxide yield carboxylic acids. ...
Organic Pathways
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
102 Lab 7 Esters Fall05
... and/or ease of purification at the end of the reaction. In this reaction we will add an excess of the acid. Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, ...
... and/or ease of purification at the end of the reaction. In this reaction we will add an excess of the acid. Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, ...
chemistry important question i
... (b) Arrange the following compounds in increasing order of their reactivity towards HCN: Acetaldehyde, Acetone, Di-tert-butyl ketone. (C) Arrange the following compounds in increasing order of acid strength: Benzoic acid, 4-Nitrobenzoic acid, 4-Methoxybenzoic acid. 34. (a) Write the mechanism of hyd ...
... (b) Arrange the following compounds in increasing order of their reactivity towards HCN: Acetaldehyde, Acetone, Di-tert-butyl ketone. (C) Arrange the following compounds in increasing order of acid strength: Benzoic acid, 4-Nitrobenzoic acid, 4-Methoxybenzoic acid. 34. (a) Write the mechanism of hyd ...
Unit 2 Review: Answers: Review for Organic Chemistry Unit Test 2
... butanone (a ketone). An oxidizing agent such as KMnO4 or Na2Cr2O7 will change colour to indicate the reaction • 2-methyl-2-propanol is a tertiary alcohol, so it will not undergo an oxidation reaction with [O]. The oxidizing agent will not change colour b) cyclopentane and cyclopentene: • cyclopentan ...
... butanone (a ketone). An oxidizing agent such as KMnO4 or Na2Cr2O7 will change colour to indicate the reaction • 2-methyl-2-propanol is a tertiary alcohol, so it will not undergo an oxidation reaction with [O]. The oxidizing agent will not change colour b) cyclopentane and cyclopentene: • cyclopentan ...
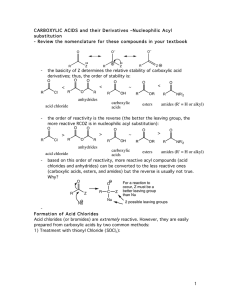
carboxylic acids esters amides (R
... OH R OR' -To drive the reaction to completion, excess alcohol must be used or water must be removed as it is formed. See Mechanism 22.6. - esterification of a carboxylic acid occurs in the presence of an acid but not in the presence of a base (since a carboxylate ion results under basic conditions). ...
... OH R OR' -To drive the reaction to completion, excess alcohol must be used or water must be removed as it is formed. See Mechanism 22.6. - esterification of a carboxylic acid occurs in the presence of an acid but not in the presence of a base (since a carboxylate ion results under basic conditions). ...
Overview of the Reactions of Carbonyl Compounds
... the tetrahedral intermediate can either be protonated to form an alcohol (NaBH4, LiAlH4, or Grignard Reduction) or a non-bonded e- pair on the nucleophile can be used to form a second bond to the carbonyl carbon. The new bond formation causes expulsion of the carbonyl oxygen as H2O. ...
... the tetrahedral intermediate can either be protonated to form an alcohol (NaBH4, LiAlH4, or Grignard Reduction) or a non-bonded e- pair on the nucleophile can be used to form a second bond to the carbonyl carbon. The new bond formation causes expulsion of the carbonyl oxygen as H2O. ...
Silicon hydrides in organic synthesis
... selective reduction of two important classes of functional groups: allylic heterosubstituents and Michael acceptors. The key details are summarized below. Allylic reductions. In our initial studies we discovered that a combination of the nonnucleophilic hydride donor, tributyltin hydride, and a solu ...
... selective reduction of two important classes of functional groups: allylic heterosubstituents and Michael acceptors. The key details are summarized below. Allylic reductions. In our initial studies we discovered that a combination of the nonnucleophilic hydride donor, tributyltin hydride, and a solu ...
Lecture 13a - University of California, Los Angeles
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
... • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A.G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/ThO2/MgO/Silica gel at 170-200 oC at 1 atm • ...
Organic 2 PPT
... Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water ...
... Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water ...
Chemistry of Nitrogen-containing Organic
... 2. Draw a reaction mechanism for an acyl chloride with 3 carbon atoms reacting with ethylamine. 3. What is this type of reaction called? 4. What type of organic compound is the product? ...
... 2. Draw a reaction mechanism for an acyl chloride with 3 carbon atoms reacting with ethylamine. 3. What is this type of reaction called? 4. What type of organic compound is the product? ...
EXPERIMENT 3: The Grignard Reaction: Synthesis of
... The reactions involved in the synthesis of complex organic molecules can commonly be categorized into either functional group interconversions or skeleton building reactions. The latter category, primarily those involving carbon-carbon bond formations, is most important in anabolic organic synthesis ...
... The reactions involved in the synthesis of complex organic molecules can commonly be categorized into either functional group interconversions or skeleton building reactions. The latter category, primarily those involving carbon-carbon bond formations, is most important in anabolic organic synthesis ...
Oxidation of Cyclohexanol
... into cyclohexanone. Bleach solution will be added to a mixture of cyclohexanol and acetic acid. No additional solvent will be used, so the only solvent will be water from bleach solution. This oxidation reaction is exothermic, which means it releases heat to the environment as the reaction progresse ...
... into cyclohexanone. Bleach solution will be added to a mixture of cyclohexanol and acetic acid. No additional solvent will be used, so the only solvent will be water from bleach solution. This oxidation reaction is exothermic, which means it releases heat to the environment as the reaction progresse ...
INTRODUCING ALDEHYDES AND KETONES
... nucleophile is a negatively charged ion (for example, a cyanide ion, CN-), or a slightly negatively charged part of a molecule (for example, the lone pair on a nitrogen atom in ammonia, NH3). During the reaction, the carbon-oxygen double bond gets broken. The net effect of all this is that the carbo ...
... nucleophile is a negatively charged ion (for example, a cyanide ion, CN-), or a slightly negatively charged part of a molecule (for example, the lone pair on a nitrogen atom in ammonia, NH3). During the reaction, the carbon-oxygen double bond gets broken. The net effect of all this is that the carbo ...
Set 1 - ExamResults.net
... 33. a) Explain the mechanism involved in the conversion of ethanol into ethene. ...
... 33. a) Explain the mechanism involved in the conversion of ethanol into ethene. ...
436
... Give an equation for a crossed cannizzaro reaction, give a mechanism, and explain why does it follow such a path ? ...
... Give an equation for a crossed cannizzaro reaction, give a mechanism, and explain why does it follow such a path ? ...
Wolff–Kishner reduction

The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.In general, the reaction mechanism first involves the in situ generation of a hydrazone by condensation of hydrazine with the ketone or aldehyde substrate. Sometimes it is however advantageous to use a pre-formed hydrazone as substrate (see modifications). The hydrazone is deprotonated by alkoxide base followed by a concerted, rate-determining step in which a diimide anion is formed. Collapse of this alkyldiimde with loss of N2 leads to formation of an alkylanion which can be protonated by solvent to give the desired product.Because the Wolff–Kishner reduction requires highly basic conditions, it is unsuitable for base-sensitive substrates. However, this method can be superior over the related Clemmensen reduction for acid-sensitive compounds such as pyrroles and for high-molecular weight compounds.