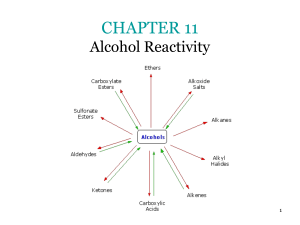
3.9alcohol rxns
... The major product is the one that results when the hydrogen is removed from the carbon atom with the smallest number of hydrogen atoms. “The poor get ...
... The major product is the one that results when the hydrogen is removed from the carbon atom with the smallest number of hydrogen atoms. “The poor get ...
Chapter 13 - WebAssign
... to produce more C-H bonds. However, multiple (double or triple) bonds are unsaturated because an H2 molecule can add across a multiple bond to form two C-H bonds. For example, ethene can be hydrogenated to ethane: H2C=CH2 + H2 → H3C-CH3. Thus, alkenes are unsaturated. Molecules with many double bond ...
... to produce more C-H bonds. However, multiple (double or triple) bonds are unsaturated because an H2 molecule can add across a multiple bond to form two C-H bonds. For example, ethene can be hydrogenated to ethane: H2C=CH2 + H2 → H3C-CH3. Thus, alkenes are unsaturated. Molecules with many double bond ...
CHAPTER 9 Further Reactions of Alcohols and the Chemistry of
... Conversion of Alcohols to Alkyl sulfonates creates a good leaving group for subsequent displacement by an anionic nucleophile ...
... Conversion of Alcohols to Alkyl sulfonates creates a good leaving group for subsequent displacement by an anionic nucleophile ...
Organic Reactions
... Organic Reactions Why? Many organic reactions lead to products we use everyday. Organic reactions can be categorized by looking at the reactants used and the products formed. Soap, alcohol, fragrances, flavors, and flames in your gas barbeque are all products of organic reactions. ...
... Organic Reactions Why? Many organic reactions lead to products we use everyday. Organic reactions can be categorized by looking at the reactants used and the products formed. Soap, alcohol, fragrances, flavors, and flames in your gas barbeque are all products of organic reactions. ...
Brominations and Alkene Synthesis CHM 233 Review
... Example problems: give the major organic products of the following reactions ...
... Example problems: give the major organic products of the following reactions ...
CHM 331 : General Organic Chemistry
... Example problems: give the major organic products of the following reactions ...
... Example problems: give the major organic products of the following reactions ...
Organic Functional Groups Organic Functional Groups
... question as to carbonyl placement. • Parent chains are named by dropping the final “e” from the name of the corresponding hydrocarbon and adding “al” for aldehydes or “one” for ketones. ...
... question as to carbonyl placement. • Parent chains are named by dropping the final “e” from the name of the corresponding hydrocarbon and adding “al” for aldehydes or “one” for ketones. ...
Oxidation of alcohol to carboxylic acid under mild acidic condition
... constipation and irritable bowel syndrome[4]. In this paper, we report the Oxidation of Corey lactone, which is sensitive to basic condition, to corresponding carboxylic acid with sodiumperiodide/sodiumchloride/water [5] in presence of TEMPO catalyst under biphasic reaction. The acid is further reac ...
... constipation and irritable bowel syndrome[4]. In this paper, we report the Oxidation of Corey lactone, which is sensitive to basic condition, to corresponding carboxylic acid with sodiumperiodide/sodiumchloride/water [5] in presence of TEMPO catalyst under biphasic reaction. The acid is further reac ...
CH 10
... • In organic chemistry, we say that oxidation occurs when a carbon or hydrogen that is connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen – Not defined as loss of electrons by an atom as in inorganic chemistry ...
... • In organic chemistry, we say that oxidation occurs when a carbon or hydrogen that is connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen – Not defined as loss of electrons by an atom as in inorganic chemistry ...
Origin of the Diastereoselection in the Indium
... Optimization of the reaction condition for the In-mediated addition of the chloroallylic sulfone (E)-1a to benzaldehyde (2a, R ) Ph in Scheme 1) has been studied first. Anhydrous THF and CH2Cl2 were not able to solvate indium, and the reaction did not proceed at all in these solvents even at reflux ...
... Optimization of the reaction condition for the In-mediated addition of the chloroallylic sulfone (E)-1a to benzaldehyde (2a, R ) Ph in Scheme 1) has been studied first. Anhydrous THF and CH2Cl2 were not able to solvate indium, and the reaction did not proceed at all in these solvents even at reflux ...
Organic Reactions
... Formation of free radicals often results in chain reactions – reaction keeps occurring until all reactant is used up. See polymerization notes form mechanism. ...
... Formation of free radicals often results in chain reactions – reaction keeps occurring until all reactant is used up. See polymerization notes form mechanism. ...
Heck Reactions
... The Complex. Among Pd(0) and Pd(II) complexes commonly used are Pd(PPh3)4, Pd2(dba)2, and Pd2(dba)2CHCl3. Pd(PPh3)4 should be stored cold and under inert gas; the dibenzylideneacetone complexes are more stable catalyst precursors. Both phosphine structure and phosphine/Pd ratio effect catalyst struc ...
... The Complex. Among Pd(0) and Pd(II) complexes commonly used are Pd(PPh3)4, Pd2(dba)2, and Pd2(dba)2CHCl3. Pd(PPh3)4 should be stored cold and under inert gas; the dibenzylideneacetone complexes are more stable catalyst precursors. Both phosphine structure and phosphine/Pd ratio effect catalyst struc ...
Mechanism
... The Henry Reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. It is similar to the Aldol Addition, and also referred to as the Nitro Aldol Reaction. If acidic protons are available (i.e. when R = H), the products tend to eliminate water to give nitro ...
... The Henry Reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. It is similar to the Aldol Addition, and also referred to as the Nitro Aldol Reaction. If acidic protons are available (i.e. when R = H), the products tend to eliminate water to give nitro ...
Chapter 23 Functional Groups
... of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? –Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 –Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 ...
... of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? –Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 –Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 ...
Chapter 19. Aldehydes and Ketones
... Addition of amines with an atom containing a lone pair of electrons on the adjacent atom occurs very readily, giving useful, stable imines For example, hydroxylamine forms oximes and 2,4dinitrophenylhydrazine readily forms 2,4dinitrophenylhydrazones ...
... Addition of amines with an atom containing a lone pair of electrons on the adjacent atom occurs very readily, giving useful, stable imines For example, hydroxylamine forms oximes and 2,4dinitrophenylhydrazine readily forms 2,4dinitrophenylhydrazones ...
... and they usually require high temperatures and/or long react ion times, and side reactions, such as isomerization, epimerization and rearrangements may be induced by the alkaline conditions. Furthermore, high temperatures are not only detrimental to certain functional groups, but also to the control ...
Chapter 1
... Aldol Condensation • Self-addition or condensation • Uses two molecules of the same aldehyde or ketone • The a carbon of the second molecule adds to the carbonyl carbon of the first molecule • Strong base such as hydroxide catalyzes the reaction • Very complex reaction occurring in multiple ...
... Aldol Condensation • Self-addition or condensation • Uses two molecules of the same aldehyde or ketone • The a carbon of the second molecule adds to the carbonyl carbon of the first molecule • Strong base such as hydroxide catalyzes the reaction • Very complex reaction occurring in multiple ...
Aldehydes and Ketones
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
Chapter 1 - dan
... Aldol Condensation • Self-addition or condensation • Uses two molecules of the same aldehyde or ketone • The a carbon of the second molecule adds to the carbonyl carbon of the first molecule • Strong base such as hydroxide catalyzes the reaction • Very complex reaction occurring in multiple ...
... Aldol Condensation • Self-addition or condensation • Uses two molecules of the same aldehyde or ketone • The a carbon of the second molecule adds to the carbonyl carbon of the first molecule • Strong base such as hydroxide catalyzes the reaction • Very complex reaction occurring in multiple ...
File
... • Even though they contain bonds, aromatic hydrocarbons undergo substitution more readily than addition (because of delocalization of bonds). • Example: if benzene is treated with nitric acid in the presence of sulfuric acid (catalyst), nitrobenzene is produced. ...
... • Even though they contain bonds, aromatic hydrocarbons undergo substitution more readily than addition (because of delocalization of bonds). • Example: if benzene is treated with nitric acid in the presence of sulfuric acid (catalyst), nitrobenzene is produced. ...
Project Overview
... Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 62 ...
... Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (–CN) group Ch. 12 - 62 ...
Chapter 26 Functional Groups and Organic Reactions
... alkane name; add ending of -ol, number the position of -OH –parent is the longest chain that contains the carbon with the hydroxyl attached. ...
... alkane name; add ending of -ol, number the position of -OH –parent is the longest chain that contains the carbon with the hydroxyl attached. ...
The Acid-Catalyzed Reaction of Acetic
... should be able to say from the density data in your Chemical Data Table, but you can do a drop test to be sure!) Remove the aqueous layer and add another 2 mL of 5% sodium bicarbonate solution. Gently mix the two layers by pipet mixing, and again remove the aqueous layer. Make sure all of the acid h ...
... should be able to say from the density data in your Chemical Data Table, but you can do a drop test to be sure!) Remove the aqueous layer and add another 2 mL of 5% sodium bicarbonate solution. Gently mix the two layers by pipet mixing, and again remove the aqueous layer. Make sure all of the acid h ...
Aldehydes & Ketones
... • Oxidation of a secondary alcohol yields a ketone. • Tertiary alcohols do not react under these conditions. ...
... • Oxidation of a secondary alcohol yields a ketone. • Tertiary alcohols do not react under these conditions. ...
Wolff–Kishner reduction

The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.In general, the reaction mechanism first involves the in situ generation of a hydrazone by condensation of hydrazine with the ketone or aldehyde substrate. Sometimes it is however advantageous to use a pre-formed hydrazone as substrate (see modifications). The hydrazone is deprotonated by alkoxide base followed by a concerted, rate-determining step in which a diimide anion is formed. Collapse of this alkyldiimde with loss of N2 leads to formation of an alkylanion which can be protonated by solvent to give the desired product.Because the Wolff–Kishner reduction requires highly basic conditions, it is unsuitable for base-sensitive substrates. However, this method can be superior over the related Clemmensen reduction for acid-sensitive compounds such as pyrroles and for high-molecular weight compounds.