
Organic Chemistry – Summary of Reactions and Conditions
... contain copper (II) complexes. These complexes enable Cu (II) to remain in solution in the presence of alkali. Ketones are resistant to oxidation and do not react with Benedict's solution. Tollen's reagent (ammoniacal silver nitrate) (not a preparative method) Prepared by adding excess ammonia solut ...
... contain copper (II) complexes. These complexes enable Cu (II) to remain in solution in the presence of alkali. Ketones are resistant to oxidation and do not react with Benedict's solution. Tollen's reagent (ammoniacal silver nitrate) (not a preparative method) Prepared by adding excess ammonia solut ...
Chemistry 235, Winter 2008 Name: General rules:
... • Hydrogen in an organic compound is assigned an oxidation number of +1. • Oxygen in an organic compound generally is assigned an oxidation number of –2. • The sum of all of the oxidation numbers of atoms in a neutral compound should be zero. The sum of all of the oxidation numbers of atoms in an io ...
... • Hydrogen in an organic compound is assigned an oxidation number of +1. • Oxygen in an organic compound generally is assigned an oxidation number of –2. • The sum of all of the oxidation numbers of atoms in a neutral compound should be zero. The sum of all of the oxidation numbers of atoms in an io ...
Thiobenzoate Photochemistry
... equatorial -H should disfavor the H-abstraction route, but it may also affect the cyclization with methylene chloride. The last substrate (9) is a derivative of a vicinal trans-diol. The bicyclic ring structure should be inflexible enough to prevent -H abstraction. One of these substrates will be ...
... equatorial -H should disfavor the H-abstraction route, but it may also affect the cyclization with methylene chloride. The last substrate (9) is a derivative of a vicinal trans-diol. The bicyclic ring structure should be inflexible enough to prevent -H abstraction. One of these substrates will be ...
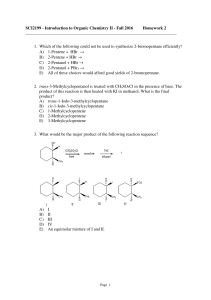
SCI2199 - Introduction to Organic Chemistry II
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
Exam 3 Key - Chemistry
... 4. (4) One of the following is not true about diborane (BH3). Which one? a) it is a reducing agent b) it reacts with carboxylic acids to form alcohols c) it does not react with nitro groups d) it is essentially a source of hydride (H-) e) it is an electron deficient species 5. (4) One of the followi ...
... 4. (4) One of the following is not true about diborane (BH3). Which one? a) it is a reducing agent b) it reacts with carboxylic acids to form alcohols c) it does not react with nitro groups d) it is essentially a source of hydride (H-) e) it is an electron deficient species 5. (4) One of the followi ...
An Efficient Method for Selective Deprotection of Trimethylsilyl
... under solvent-free conditions with this reagent failed in the absence of catalyst, the effect of several Lewis acids such as ZnCl2, FeCl3, FeBr3, SnCl2, SnCl4, CuCl2, BiCl3, AlBr3, and AlCl3 were also examined under solvent-free conditions. Surprisingly, only AlCl3 was shown to be effective catalyst ...
... under solvent-free conditions with this reagent failed in the absence of catalyst, the effect of several Lewis acids such as ZnCl2, FeCl3, FeBr3, SnCl2, SnCl4, CuCl2, BiCl3, AlBr3, and AlCl3 were also examined under solvent-free conditions. Surprisingly, only AlCl3 was shown to be effective catalyst ...
Synthesizing Organic Compounds
... 1-butanol is difficult to prepare from the reactions we have studied so far. The addition reaction of H2O to either 1-butene or 2-butene would produce predominantly 2-butanol, as predicted by Markovnikov’s rule. CH2 ...
... 1-butanol is difficult to prepare from the reactions we have studied so far. The addition reaction of H2O to either 1-butene or 2-butene would produce predominantly 2-butanol, as predicted by Markovnikov’s rule. CH2 ...
Review and New - ChemConnections
... • Treatment of 1-propanol with K2Cr2O7, H2SO4, and heat will produce: ...
... • Treatment of 1-propanol with K2Cr2O7, H2SO4, and heat will produce: ...
cellulose
... CH] groups leaves the cyclic ring, which is chal"acterised by the peaks at masses 133 (I) and 148 (11). These I"esults support the given cyclic structure. The fragmentation of the cyclic ring proceeds through the stepwise elimination of the CH2-groups leaving the C-Si-O part with the mass 104. The f ...
... CH] groups leaves the cyclic ring, which is chal"acterised by the peaks at masses 133 (I) and 148 (11). These I"esults support the given cyclic structure. The fragmentation of the cyclic ring proceeds through the stepwise elimination of the CH2-groups leaving the C-Si-O part with the mass 104. The f ...
Oxidation and Reduction Reactions
... As noted before, this reaction is not particularly chemoselective. As long as there’s a (non-aromatic) bond, hydrogen will add. Draw the major organic product of each hydrogenation reaction: H2 Pd/C ...
... As noted before, this reaction is not particularly chemoselective. As long as there’s a (non-aromatic) bond, hydrogen will add. Draw the major organic product of each hydrogenation reaction: H2 Pd/C ...
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS 1 ORGANIC
... 13. When HX adds to an alkyne or alkene, describe how you know which carbon the H adds to and which carbon the X adds to and why. 14. When X2 adds to an alkene, where do the X’s end up? 15. a.) When 1 eq. X2 adds to an alkyne, where do the X’s end up? b.) When excess X2 adds to an alkyne, where do t ...
... 13. When HX adds to an alkyne or alkene, describe how you know which carbon the H adds to and which carbon the X adds to and why. 14. When X2 adds to an alkene, where do the X’s end up? 15. a.) When 1 eq. X2 adds to an alkyne, where do the X’s end up? b.) When excess X2 adds to an alkyne, where do t ...
Synopsis
... diastereomeric sulfilimines behave in a stereoconvergent fashion and afford products with the same configuration at carbon. An efficient route to αhydroxy-β-amino acid derivatives AHDA and AHPBA was developed using a common advanced intermediate. The methodology provides aminoalcohol derivatives wit ...
... diastereomeric sulfilimines behave in a stereoconvergent fashion and afford products with the same configuration at carbon. An efficient route to αhydroxy-β-amino acid derivatives AHDA and AHPBA was developed using a common advanced intermediate. The methodology provides aminoalcohol derivatives wit ...
Naming Ethers Naming Ethers
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
... • Skunks use thiols as a defense mechanism: (E)-2butene-1-thiol, 3-methyl-1-butanethiol, and 2quinolinemethanethiol, and acetate thioesters of these. • Methanethiol is added to natural gas (methane) so that gas leaks can be detected. • The hydrosulfide ion (HS–) is a strong nucleophile and a weak ...
chemistry ch4 - The Student Room
... The reaction of this compound with hot aqueous sodium hydroxide shows, as with chlorobenzene, that it is difficult to remove the chlorine in this way, by hydrolysis. Explain why this is relatively difficult to achieve when compared to the alkaline hydrolysis of 1-chlorobutane. In your answer you sho ...
... The reaction of this compound with hot aqueous sodium hydroxide shows, as with chlorobenzene, that it is difficult to remove the chlorine in this way, by hydrolysis. Explain why this is relatively difficult to achieve when compared to the alkaline hydrolysis of 1-chlorobutane. In your answer you sho ...
New synthetic methodologies based on active transition metals*
... amount of a polymer-supported arene, promoted the homocoupling of various aryl iodides under relatively mild reaction conditions (THF reflux) [13]. The presence of an organic base was fundamental for the reaction to occur, lithium ethoxide, generated in situ, giving the best results. Bathophenanthro ...
... amount of a polymer-supported arene, promoted the homocoupling of various aryl iodides under relatively mild reaction conditions (THF reflux) [13]. The presence of an organic base was fundamental for the reaction to occur, lithium ethoxide, generated in situ, giving the best results. Bathophenanthro ...
Document
... on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and change the “e” ending of the parent alkane to the suffix “oic acid”. If the COOH is bonded to a ring, name the ring and add the words “carboxylic acid”. Numb ...
... on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and change the “e” ending of the parent alkane to the suffix “oic acid”. If the COOH is bonded to a ring, name the ring and add the words “carboxylic acid”. Numb ...
Carboxylic Acids and the Acidity of the O—H Bond
... on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and change the “e” ending of the parent alkane to the suffix “oic acid”. If the COOH is bonded to a ring, name the ring and add the words “carboxylic acid”. Numb ...
... on whether the carboxy group is bonded to a chain or a ring. If the COOH is bonded to a chain, find the longest chain containing the COOH, and change the “e” ending of the parent alkane to the suffix “oic acid”. If the COOH is bonded to a ring, name the ring and add the words “carboxylic acid”. Numb ...
ch13[1].
... • For an aldehyde, change the suffix from -e to -al. • For an unsaturated aldehyde, show the carbon-carbon double bond by changing the infix from -an- to -en-; the location of the suffix determines the numbering pattern. • For a cyclic molecule in which -CHO is bonded to the ring, add the suffix -ca ...
... • For an aldehyde, change the suffix from -e to -al. • For an unsaturated aldehyde, show the carbon-carbon double bond by changing the infix from -an- to -en-; the location of the suffix determines the numbering pattern. • For a cyclic molecule in which -CHO is bonded to the ring, add the suffix -ca ...
Document
... Introduction to Alkyne Reactions—Acetylide anions • Because sp hybridized C—H bonds are more acidic than sp2 and sp3 hybridized C—H bonds, terminal alkynes are readily deprotonated with strong base in a BrØnstedLowry acid-base reaction. The resulting ion is called the ...
... Introduction to Alkyne Reactions—Acetylide anions • Because sp hybridized C—H bonds are more acidic than sp2 and sp3 hybridized C—H bonds, terminal alkynes are readily deprotonated with strong base in a BrØnstedLowry acid-base reaction. The resulting ion is called the ...
ORGANIC REACTIONS IN A CLAY MICROENVIRONMENT
... R _ C H 2 _ C H 2 _ O H AIbentonit%R _ C H 2 _ C H 2 _ O _ C H 2 _ C H 2 _ R + H20 Following the successful use of ion-exchanged bentonites for proton addition to alkenes it seemed obvious to examine their reactivity in other acid-catalysed reactions. Accordingly their reactivity in the dehydration ...
... R _ C H 2 _ C H 2 _ O H AIbentonit%R _ C H 2 _ C H 2 _ O _ C H 2 _ C H 2 _ R + H20 Following the successful use of ion-exchanged bentonites for proton addition to alkenes it seemed obvious to examine their reactivity in other acid-catalysed reactions. Accordingly their reactivity in the dehydration ...
18.10 CONJUGATE ADDITIONS
... The overall result of a conjugate addition is the addition of a proton and a nucleophile to the CC double bond. However, this reaction differs greatly from the additions discussed in Chapter 11, in which the electrophile adds first. Here, the nucleophile adds in the first step. This reaction does no ...
... The overall result of a conjugate addition is the addition of a proton and a nucleophile to the CC double bond. However, this reaction differs greatly from the additions discussed in Chapter 11, in which the electrophile adds first. Here, the nucleophile adds in the first step. This reaction does no ...
Chapter 9
... The alkyne can also be oxidized similar to alkene reactions with transition metals ...
... The alkyne can also be oxidized similar to alkene reactions with transition metals ...
SYNOPSIS
... Chapter III: Lithium perchlorate catalyzed reactions of indoles: An expeditious synthesis of Bis (indolyl) methanes. The acid-catalyzed reaction of electron-rich heterocyclic compounds with pdimethylamino benzaldehyde is known as Ehrlich test for -electron excessive heterocycles such as pyroles and ...
... Chapter III: Lithium perchlorate catalyzed reactions of indoles: An expeditious synthesis of Bis (indolyl) methanes. The acid-catalyzed reaction of electron-rich heterocyclic compounds with pdimethylamino benzaldehyde is known as Ehrlich test for -electron excessive heterocycles such as pyroles and ...
Experiments
... two layers, green and ochre. Upon the addition of water (removing the H+ from the HSO3- group), there is a milky ppt. A solution of fuming sulphuric acid is used here to provide the attacking molecule, SO3. Refluxing for several hours is sometimes necessary with benzene, though not with methyl or ...
... two layers, green and ochre. Upon the addition of water (removing the H+ from the HSO3- group), there is a milky ppt. A solution of fuming sulphuric acid is used here to provide the attacking molecule, SO3. Refluxing for several hours is sometimes necessary with benzene, though not with methyl or ...
Wolff–Kishner reduction

The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.In general, the reaction mechanism first involves the in situ generation of a hydrazone by condensation of hydrazine with the ketone or aldehyde substrate. Sometimes it is however advantageous to use a pre-formed hydrazone as substrate (see modifications). The hydrazone is deprotonated by alkoxide base followed by a concerted, rate-determining step in which a diimide anion is formed. Collapse of this alkyldiimde with loss of N2 leads to formation of an alkylanion which can be protonated by solvent to give the desired product.Because the Wolff–Kishner reduction requires highly basic conditions, it is unsuitable for base-sensitive substrates. However, this method can be superior over the related Clemmensen reduction for acid-sensitive compounds such as pyrroles and for high-molecular weight compounds.















![ch13[1].](http://s1.studyres.com/store/data/008194698_1-d188c504eac7b7806e762a2340484910-300x300.png)






