Heck Reactions
... natural product total synthesis." Chem. Rev. 2003, 103, 2945-2963. Beletskaya, I. P.; Cheprakov, A. V. "The Heck reaction as a sharpening stone of palladium catalysis." Chem. Rev. 2000, 100, 3009-3066. ...
... natural product total synthesis." Chem. Rev. 2003, 103, 2945-2963. Beletskaya, I. P.; Cheprakov, A. V. "The Heck reaction as a sharpening stone of palladium catalysis." Chem. Rev. 2000, 100, 3009-3066. ...
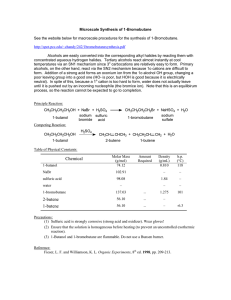
Synthesis of 1
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
Lecture6-Organometallic Chemistry
... • Principle of Microscopic Reversibility • If a certain series of steps constitutes the mechanism of a forward reaction, the mechanism of the reverse reaction is given by the same steps traversed backwards. (applies only to thermal reactions and not-photochemical reactions) • The sequence of transi ...
... • Principle of Microscopic Reversibility • If a certain series of steps constitutes the mechanism of a forward reaction, the mechanism of the reverse reaction is given by the same steps traversed backwards. (applies only to thermal reactions and not-photochemical reactions) • The sequence of transi ...
Document
... Synthesis Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to ...
... Synthesis Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to ...
Name
... a. Theoretical yield b. Percentage yield c. Mole ratio d. Actual yield 14. For the reaction Cl2 + 2KBr → 2KCl +Br2, calculate the percentage yield if 200g of chlorine react with excess potassium bromide to produce 410g of bromine. a. 73.4% b. 82.1% c. 91.0% d. 98.9% 15. For the reaction Mg + 2HCl → ...
... a. Theoretical yield b. Percentage yield c. Mole ratio d. Actual yield 14. For the reaction Cl2 + 2KBr → 2KCl +Br2, calculate the percentage yield if 200g of chlorine react with excess potassium bromide to produce 410g of bromine. a. 73.4% b. 82.1% c. 91.0% d. 98.9% 15. For the reaction Mg + 2HCl → ...
CHMY_271_practice_exam_3
... 11. (6 pt) If the following alkyl halide were to undergo elimination, predict the major product in each case, and explain your answer. You do not need to draw out the mechanism, but knowing the mechanism will help you to predict reasonable products. Br ...
... 11. (6 pt) If the following alkyl halide were to undergo elimination, predict the major product in each case, and explain your answer. You do not need to draw out the mechanism, but knowing the mechanism will help you to predict reasonable products. Br ...
Macromolecules
... 9. _________________fatty acids contain all of the __________________ they can hold. There are no carbon to carbon ________________bonds in saturated fatty acids. Saturated fatty acids are typical of _________________ fats and are believed to cause blockage of ___________________ which can lead to s ...
... 9. _________________fatty acids contain all of the __________________ they can hold. There are no carbon to carbon ________________bonds in saturated fatty acids. Saturated fatty acids are typical of _________________ fats and are believed to cause blockage of ___________________ which can lead to s ...
Chapter 8_part 1
... produces two products: 2-chloro-3-methylbutane and 2chloro-2-methylbutane Give the structure and name of the product that would be obatained from ionic addition of IBr to propene ...
... produces two products: 2-chloro-3-methylbutane and 2chloro-2-methylbutane Give the structure and name of the product that would be obatained from ionic addition of IBr to propene ...
Get PDF - Wiley Online Library
... the less hindered alkene p-face.[12] This material was subjected directly to reduction with LiAlH4 to deliver alcohol 12 in 63 % yield from 11. Finally, hydrolysis of the TBS ether and double oxidation under Swern conditions gave the targeted aldehyde 4 in 74 % yield (13 % overall from 6). Initial a ...
... the less hindered alkene p-face.[12] This material was subjected directly to reduction with LiAlH4 to deliver alcohol 12 in 63 % yield from 11. Finally, hydrolysis of the TBS ether and double oxidation under Swern conditions gave the targeted aldehyde 4 in 74 % yield (13 % overall from 6). Initial a ...
Solution 1. - TutorBreeze.com
... (vii) Ketal :- Dialkoxyalkanes are called ketals. In Ketals , the two alkoxy groups are present on the same carbon within the chain. ...
... (vii) Ketal :- Dialkoxyalkanes are called ketals. In Ketals , the two alkoxy groups are present on the same carbon within the chain. ...
CHE 322
... conditions, to make the indicated large compound? In each case show the reaction that makes the C-C or C-O bond that links the pieces. All three must be different kinds of reactions. [Caution: parts of some reaction partners are missing in the given products due to replacement or subsequent reaction ...
... conditions, to make the indicated large compound? In each case show the reaction that makes the C-C or C-O bond that links the pieces. All three must be different kinds of reactions. [Caution: parts of some reaction partners are missing in the given products due to replacement or subsequent reaction ...
Conjugate (1,4
... This synthesis is by G. H. Posner, T. P. Kogan, S. R. Haines, L. L. Frye, Tetrahedron ...
... This synthesis is by G. H. Posner, T. P. Kogan, S. R. Haines, L. L. Frye, Tetrahedron ...
10. Alkyl Halides - University of West Alabama
... 10.7 Preparing Alkyl Halides from Alcohols • Reaction of tertiary C-OH with HX is fast and effective – Add HCl or HBr gas into ether solution of tertiary alcohol • Primary and secondary alcohols react very slowly and often rearrange, so alternative methods are used ...
... 10.7 Preparing Alkyl Halides from Alcohols • Reaction of tertiary C-OH with HX is fast and effective – Add HCl or HBr gas into ether solution of tertiary alcohol • Primary and secondary alcohols react very slowly and often rearrange, so alternative methods are used ...
Working with Hazardous Chemicals
... care should be taken to keep the liquid off the skin. Methyl iodide, in high concentrations for short periods or in low concentrations for long periods, can cause serious toxic effects in the central nervous system. Accordingly, the American Conference of Governmental Industrial Hygienists3 has set ...
... care should be taken to keep the liquid off the skin. Methyl iodide, in high concentrations for short periods or in low concentrations for long periods, can cause serious toxic effects in the central nervous system. Accordingly, the American Conference of Governmental Industrial Hygienists3 has set ...
BOC-ON - Sigma
... This reagent offers a distinct advantage over t-BOC azide which can require reaction temperatures of 50-60°C (t-BOC azide is thermally unstable and decomposes with apparent detonation at temperatures above 80°C).1 The oxime by-product can be easily and completely removed from the reaction mixture by ...
... This reagent offers a distinct advantage over t-BOC azide which can require reaction temperatures of 50-60°C (t-BOC azide is thermally unstable and decomposes with apparent detonation at temperatures above 80°C).1 The oxime by-product can be easily and completely removed from the reaction mixture by ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... 1. name like alkenes, but no cis/trans or E/Z 2. find longest chain containing funct. grp and # so funct grp # smallest 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numberi ...
... 1. name like alkenes, but no cis/trans or E/Z 2. find longest chain containing funct. grp and # so funct grp # smallest 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numberi ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.