Microsoft Word
... chloride, 2-trimethylsiloxyfuran reacted with various aldehydes, to give the corresponding butenolides in high yields. Recent reports on 2-trimethylsiloxyfuran show that it has promise as a masked butenolide. However, in order to exploit in synthesis the appropriate conditions need to be found for c ...
... chloride, 2-trimethylsiloxyfuran reacted with various aldehydes, to give the corresponding butenolides in high yields. Recent reports on 2-trimethylsiloxyfuran show that it has promise as a masked butenolide. However, in order to exploit in synthesis the appropriate conditions need to be found for c ...
Lesson 4 halogenoalkanes
... A study of the reaction kinetics show that the reaction is first order w.r.t. the halogenoalkane but zero order w.r.t. water. i.e. Rate = k[(CH3)3CBr(l) ] ...
... A study of the reaction kinetics show that the reaction is first order w.r.t. the halogenoalkane but zero order w.r.t. water. i.e. Rate = k[(CH3)3CBr(l) ] ...
Alkynes
... Similar to formation of an anti-Markovnikov alcohol from an alkene Step 1, Internal Alkyne: addition to the alkyne with little or no regioselectivity issue. ...
... Similar to formation of an anti-Markovnikov alcohol from an alkene Step 1, Internal Alkyne: addition to the alkyne with little or no regioselectivity issue. ...
Q 1: Molecular formula of BHA is
... ( A) In alcohols - OH group always present at the end of the chain. ( B) Alcohols may contain one or more groups. ( C) butane-1-ol has higher boiling point than 2-methyl ...
... ( A) In alcohols - OH group always present at the end of the chain. ( B) Alcohols may contain one or more groups. ( C) butane-1-ol has higher boiling point than 2-methyl ...
aldehydes powerpoint
... • Tollens' reagent is a chemical reagent most commonly used to determine whether a known carbonyl-containing compound is an aldehyde or a ketone. It is usually ammoniacal silver nitrate, but can also be other mixtures, as long as aqueous diamminesilver(I) complex is present. It was named after its d ...
... • Tollens' reagent is a chemical reagent most commonly used to determine whether a known carbonyl-containing compound is an aldehyde or a ketone. It is usually ammoniacal silver nitrate, but can also be other mixtures, as long as aqueous diamminesilver(I) complex is present. It was named after its d ...
Chemistry 3719L – Week 9 Reduction of Benzil with Sodium
... • In a 125 mL Erlenmeyer flask add benzil (1.5 g) and 95% ethanol (15 mL). Cool the flask under running water such that the benzyl forms a fine suspension in the ethanol. • Weigh out sodium borohydride (0.3 g – careful, this fine powder is dangerous if accidentally inhaled) and add this to the react ...
... • In a 125 mL Erlenmeyer flask add benzil (1.5 g) and 95% ethanol (15 mL). Cool the flask under running water such that the benzyl forms a fine suspension in the ethanol. • Weigh out sodium borohydride (0.3 g – careful, this fine powder is dangerous if accidentally inhaled) and add this to the react ...
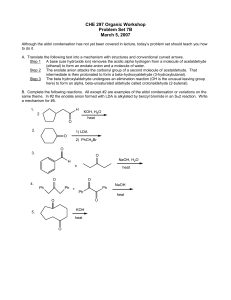
CHE 297 Organic Workshop
... Although the aldol condensation has not yet been covered in lecture, today’s problem set should teach you how to do it. A. Translate the following text into a mechanism with structures and conventional curved arrows. Step 1 A base (use hydroxide ion) removes the acidic alpha hydrogen from a molecule ...
... Although the aldol condensation has not yet been covered in lecture, today’s problem set should teach you how to do it. A. Translate the following text into a mechanism with structures and conventional curved arrows. Step 1 A base (use hydroxide ion) removes the acidic alpha hydrogen from a molecule ...
Calculating Percent Yield
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
Chemical Synthesis (sat6)
... E = (M gO ∧ H2 → M g ∧ H2 O) ∧ (C ∧ O2 → CO2 )∧ (CO2 ∧ H2 O → H2 CO3 ) ∧ M gO ∧ H2 ∧ O2 ∧ C F = H2 CO3 The complete model code in LPL for this model is as follows (see [1]): Listing 1: The Model ...
... E = (M gO ∧ H2 → M g ∧ H2 O) ∧ (C ∧ O2 → CO2 )∧ (CO2 ∧ H2 O → H2 CO3 ) ∧ M gO ∧ H2 ∧ O2 ∧ C F = H2 CO3 The complete model code in LPL for this model is as follows (see [1]): Listing 1: The Model ...
reactions of the carbonyl group in aldehydes and ketones
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
Organo halides
... Reaction distinction is more selective with bromine than chlorine (1700:80:1 for 3o:2o:1o) ...
... Reaction distinction is more selective with bromine than chlorine (1700:80:1 for 3o:2o:1o) ...
Microsoft Word - Open Access Repository of Indian Theses
... For discovering new peptide-based drugs, many structurally rigid nonpeptidic molecular scaffolds have been designed. Insertion of these moieties in appropriate sites, a common approach to restrict the conformational degrees of freedom in peptides, produces the specific three-dimensional structures r ...
... For discovering new peptide-based drugs, many structurally rigid nonpeptidic molecular scaffolds have been designed. Insertion of these moieties in appropriate sites, a common approach to restrict the conformational degrees of freedom in peptides, produces the specific three-dimensional structures r ...
Cholesterol Synthesis - The Center for Cholesterol Management
... In the second step, mevalonate is phosphorylated from ATP to isoprene units or isoprenoids, namely isopentyl pyrophosphate, which can isomerize or interconvert to dimethylallyl pyrophosphate ...
... In the second step, mevalonate is phosphorylated from ATP to isoprene units or isoprenoids, namely isopentyl pyrophosphate, which can isomerize or interconvert to dimethylallyl pyrophosphate ...
Research projects for Dr
... The reaction generally involves the treatment of primary or secondary alcohol with an activated sulfonium salt (usually derived from dimethyl sulfoxide, DMSO). Subsequent treatment with base gives the corresponding carbonyl aldehyde or ketone. Dr Fitch is interested in developing asymmetric variants ...
... The reaction generally involves the treatment of primary or secondary alcohol with an activated sulfonium salt (usually derived from dimethyl sulfoxide, DMSO). Subsequent treatment with base gives the corresponding carbonyl aldehyde or ketone. Dr Fitch is interested in developing asymmetric variants ...
Lecture5
... form new carbon-carbon bonds by coupling with alkyl and alkenyl chlorides, bromides, and iodides ...
... form new carbon-carbon bonds by coupling with alkyl and alkenyl chlorides, bromides, and iodides ...
This is the first exam with targeted syntheses that you
... The Wittig is unique in that the alkoxide oxygen in the tetrahedral intermediate attacks the phosphonium center forming an oxaphosphetane intermediate. Thus, the electrophile is not H+ as in the previous examples but the phosphonium center. The intermediate undergoes a reverse 2+2 process to form tr ...
... The Wittig is unique in that the alkoxide oxygen in the tetrahedral intermediate attacks the phosphonium center forming an oxaphosphetane intermediate. Thus, the electrophile is not H+ as in the previous examples but the phosphonium center. The intermediate undergoes a reverse 2+2 process to form tr ...
10. Alkyl Halides - Clayton State University
... Alkyllithium (RLi) forms from RBr and Li metal RLi (primary, secondary or tertiary alkyl, aryl or vinyl R group) reacts with copper iodide to give lithium ...
... Alkyllithium (RLi) forms from RBr and Li metal RLi (primary, secondary or tertiary alkyl, aryl or vinyl R group) reacts with copper iodide to give lithium ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.