CH 3502 4500
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
Industriel katalys
... Catalysis: definition, classification with examples, selectivity issues a) Homogeneous and heterogeneous catalysis b) Gas phase-, liquid phase-, biphasic catalysis, phase-transfer catalysis c) Metal catalysis, biocatalysis, organocatalysis Safety issues: risk and safety analysis, oxygen balance II. ...
... Catalysis: definition, classification with examples, selectivity issues a) Homogeneous and heterogeneous catalysis b) Gas phase-, liquid phase-, biphasic catalysis, phase-transfer catalysis c) Metal catalysis, biocatalysis, organocatalysis Safety issues: risk and safety analysis, oxygen balance II. ...
Dess-Martin Periodinane
... One of the mildest reagents for the selective oxidation of primary and secondary alcohols to aldehydes and ketones. High yields can be obtained at ambient temperatures, under neutral conditions, in chloroform, dichloromethane or acetonitrile: J. Org. Chem., 48, 4155 (1983); J. Am. Chem. Soc., 113, 7 ...
... One of the mildest reagents for the selective oxidation of primary and secondary alcohols to aldehydes and ketones. High yields can be obtained at ambient temperatures, under neutral conditions, in chloroform, dichloromethane or acetonitrile: J. Org. Chem., 48, 4155 (1983); J. Am. Chem. Soc., 113, 7 ...
F:\CH 361 2014\Prelab 6 2011 CH361 modified for 2014.wpd
... This Prelab is due at 1pm on Tuesday, Nov 11, for the T/R section, or Wednesday Nov 12, for the W/F section. Please read the section on the elimination of alcohols to make alkenes in your organic chemistry textbook and the rest of experiment II in your lab manual. This prelab has two ...
... This Prelab is due at 1pm on Tuesday, Nov 11, for the T/R section, or Wednesday Nov 12, for the W/F section. Please read the section on the elimination of alcohols to make alkenes in your organic chemistry textbook and the rest of experiment II in your lab manual. This prelab has two ...
New aniline photocage for carboxylic acids
... biological sciences. It was shown that they can be used for light-controlled small molecule release (NO, H2S, CO, HNO) in biological systems [1], photocontrolled targeted drug delivery [2] and in molecular imaging and cell biology [3]. Very important area for application of PPGs is organic synthesis ...
... biological sciences. It was shown that they can be used for light-controlled small molecule release (NO, H2S, CO, HNO) in biological systems [1], photocontrolled targeted drug delivery [2] and in molecular imaging and cell biology [3]. Very important area for application of PPGs is organic synthesis ...
Chem 30CL - Lecture 1c - UCLA Chemistry and Biochemistry
... • This places the peroxide function in close proximity EtO E=COOEt to the alkene function • The reaction is usually carried out at low temperatures (-20 oC), is very sensitive towards water and require up to 18 hours to complete • The yields are moderate (77 % for the reaction above) due to the incr ...
... • This places the peroxide function in close proximity EtO E=COOEt to the alkene function • The reaction is usually carried out at low temperatures (-20 oC), is very sensitive towards water and require up to 18 hours to complete • The yields are moderate (77 % for the reaction above) due to the incr ...
review sheet plus practice problems
... Why are allylic radicals more stable than other types? Draw resonance for all allylic radicals. What is the definition of oxidation? Which compound is at a higher oxidation state? Is this reaction an oxidation, reduction, or neither? Describe hydrogen bonding in alcohols, and compare alcohol polarit ...
... Why are allylic radicals more stable than other types? Draw resonance for all allylic radicals. What is the definition of oxidation? Which compound is at a higher oxidation state? Is this reaction an oxidation, reduction, or neither? Describe hydrogen bonding in alcohols, and compare alcohol polarit ...
sn2 reactions of alkyl halides
... The alkyl halides play an important role in organic synthesis. They can be easily prepared from alcohols or alkenes, among other starting materials. They in turn can be used in the synthesis of a large number of functional groups. These syntheses are often carried out by nucleophilic substitution re ...
... The alkyl halides play an important role in organic synthesis. They can be easily prepared from alcohols or alkenes, among other starting materials. They in turn can be used in the synthesis of a large number of functional groups. These syntheses are often carried out by nucleophilic substitution re ...
Microsoft Word
... Scheme 1. Synthesis of Nitrovinyl Biphenyls using Palladium catalyzed Suzuki cross coupling. A majority of these compounds, except, 26c, g and k demonstrated significant anti-proliferative activity against a majority of cell lines tested. The compounds 26e and 26f possessed anti-tubulin activity bot ...
... Scheme 1. Synthesis of Nitrovinyl Biphenyls using Palladium catalyzed Suzuki cross coupling. A majority of these compounds, except, 26c, g and k demonstrated significant anti-proliferative activity against a majority of cell lines tested. The compounds 26e and 26f possessed anti-tubulin activity bot ...
8. Chemistry of cooking
... Propanone is a widely used solvent. It can be made from propene. Using full structural formulae show the steps involved in this preparation and name the reagent used in each step. ...
... Propanone is a widely used solvent. It can be made from propene. Using full structural formulae show the steps involved in this preparation and name the reagent used in each step. ...
Drug Design
... the appropriate stereo-chemical environment so that only one enantiomer is produced. ...
... the appropriate stereo-chemical environment so that only one enantiomer is produced. ...
Slide 1
... the appropriate stereo-chemical environment so that only one enantiomer is produced. ...
... the appropriate stereo-chemical environment so that only one enantiomer is produced. ...
Synthetic route to novel asymmetric tetradentate ligands
... The proposed synthetic route afforded the desired asymmetric product with a reasonable yield. The final ligand and all the organic intermediates have been successfully characterised. This synthetic strategy may be used to obtain a new family of organic ligands suitable for coordination of different ...
... The proposed synthetic route afforded the desired asymmetric product with a reasonable yield. The final ligand and all the organic intermediates have been successfully characterised. This synthetic strategy may be used to obtain a new family of organic ligands suitable for coordination of different ...
Here is the Original File - University of New Hampshire
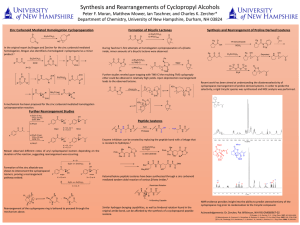
... Synthesis and Rearrangements of Cyclopropyl Alcohols Peter F. Moran, Matthew Mower, Ian Taschner, and Charles K. Zercher* Department of Chemistry, University of New Hampshire, Durham, NH 03824 ...
... Synthesis and Rearrangements of Cyclopropyl Alcohols Peter F. Moran, Matthew Mower, Ian Taschner, and Charles K. Zercher* Department of Chemistry, University of New Hampshire, Durham, NH 03824 ...
Nugget
... but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases to probe the mechanism of this ring inversion. The synthesis of the required chiral compounds will require the synthesis of the optically pure 4-amino-3-(1-hydroxyethyl) ...
... but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases to probe the mechanism of this ring inversion. The synthesis of the required chiral compounds will require the synthesis of the optically pure 4-amino-3-(1-hydroxyethyl) ...
Synthesis of (−)-Epibatidine - David A. Evans
... Although the reduction proceeded without affecting the chloropyridine ring,21 a 75:25 mixture of inseparable alcohols was obtained. The structural assignment of the major diastereomer was complicated as a result of slowly interconverting conformations as observed by 1H NMR spectroscopy, even at elev ...
... Although the reduction proceeded without affecting the chloropyridine ring,21 a 75:25 mixture of inseparable alcohols was obtained. The structural assignment of the major diastereomer was complicated as a result of slowly interconverting conformations as observed by 1H NMR spectroscopy, even at elev ...
Problem Set: Empirical and Molecular Formulas
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
2015 CH 420 Take Home Quiz 3 March 24
... could “flip” either substrate horizontally to see the other theoretical product). In the boxes above, draw an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
... could “flip” either substrate horizontally to see the other theoretical product). In the boxes above, draw an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... arrangement of the carbon atom and the remaining three groups ...
... arrangement of the carbon atom and the remaining three groups ...
CHAPTER II. A Facile Synthesis of Arylacetic Acid Derivatives via
... “Design and Synthesis of Some Biologically Active Aryl Alkanoic Acid Derivatives and Development of Novel Synthetic Methodologies” The thesis has been presented as five chapters. Chapter-I deals with general introduction on the synthesis of arylacetic acids, -hydroxy arylacetic acid (mandelic acid) ...
... “Design and Synthesis of Some Biologically Active Aryl Alkanoic Acid Derivatives and Development of Novel Synthetic Methodologies” The thesis has been presented as five chapters. Chapter-I deals with general introduction on the synthesis of arylacetic acids, -hydroxy arylacetic acid (mandelic acid) ...
Experiment 1
... A m____ of sand and table salt is given. The first step is to d____ the salt by adding suitable amount of w____. The sand is dissolved in w____ to form a salt s____ while the sand is not soluble (i______) in water and stay at the bottom of the beaker. ...
... A m____ of sand and table salt is given. The first step is to d____ the salt by adding suitable amount of w____. The sand is dissolved in w____ to form a salt s____ while the sand is not soluble (i______) in water and stay at the bottom of the beaker. ...
Whitten, Davis, and Peck, General Chemistry, 6th Edition
... Recommended CER Experiments to accompany Hornback’s Organic Chemistry, Second Edition The table below matches sections from the book with recommended CER labs. Click on the experiment title to view a PDF of each lab. Go to www.CERLabs.com to search the complete CER database and to learn more about c ...
... Recommended CER Experiments to accompany Hornback’s Organic Chemistry, Second Edition The table below matches sections from the book with recommended CER labs. Click on the experiment title to view a PDF of each lab. Go to www.CERLabs.com to search the complete CER database and to learn more about c ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.