PHENOL - Gneet's
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
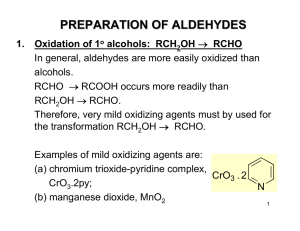
PREPARATION OF ALDEHYDES
... 2. Reduction of acyl chlorides: RCOCl → RCHO Lithium tri-t-butoxyaluminum hydride is often used for this reduction. CH3 H3C C O ...
... 2. Reduction of acyl chlorides: RCOCl → RCHO Lithium tri-t-butoxyaluminum hydride is often used for this reduction. CH3 H3C C O ...
Synthesis and Structure of Alcohols
... (1) Name the longest carbon chain bearing the –OH group. Drop the last –e from the alkane name and add –ol to obtain the root name. (2) Number the longest chain starting at the end nearest the –OH group, and designate a number for the –OH group. (Hydroxyl has greater priority than carbon-carbon mult ...
... (1) Name the longest carbon chain bearing the –OH group. Drop the last –e from the alkane name and add –ol to obtain the root name. (2) Number the longest chain starting at the end nearest the –OH group, and designate a number for the –OH group. (Hydroxyl has greater priority than carbon-carbon mult ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
Chapter 23 SG5e
... function of alanine results in formation of an overall neutral zwitterion. Thus, the carboxylate form of alanine can be thought of as being neutralized by the adjacent positively-charged ammonium ion. Problem 23.9 In what way(s) might the results of the separation and purification procedure outlined ...
... function of alanine results in formation of an overall neutral zwitterion. Thus, the carboxylate form of alanine can be thought of as being neutralized by the adjacent positively-charged ammonium ion. Problem 23.9 In what way(s) might the results of the separation and purification procedure outlined ...
Chapter 22 and 23 Study Guide
... Be able to define all boldface words from Ch. 20. How many valence electrons does a carbon atom have? How many covalent bonds can each carbon atom form? How is ethanol produced in nature? Be able to write the molecular formula of alkanes, alkenes and alkynes with 1-10 carbon atoms. Example: what is ...
... Be able to define all boldface words from Ch. 20. How many valence electrons does a carbon atom have? How many covalent bonds can each carbon atom form? How is ethanol produced in nature? Be able to write the molecular formula of alkanes, alkenes and alkynes with 1-10 carbon atoms. Example: what is ...
lecture 6 oxidative addition
... • Oxidative additions proceed by a great variety of mechanisms, however, a vacant 2e site is always required on the metal. • We can either start with a 16e complex or a 2e site must be opened up in an 18e complex by the loss of a ligand producing a 16e intermediate species. • The change in oxidation ...
... • Oxidative additions proceed by a great variety of mechanisms, however, a vacant 2e site is always required on the metal. • We can either start with a 16e complex or a 2e site must be opened up in an 18e complex by the loss of a ligand producing a 16e intermediate species. • The change in oxidation ...
Full Text - Journal of the Indian Institute of Science
... Methyl N-(triethylammoniumsulphonyl)carbamate (1), also known as Burgess reagent,1 is a mild and selective dehydrating agent, and can be successfully utilized for the preparation of alkenes from alcohols. However, it went into oblivion for nearly a decade soon after its discovery by E. M. Burgess in ...
... Methyl N-(triethylammoniumsulphonyl)carbamate (1), also known as Burgess reagent,1 is a mild and selective dehydrating agent, and can be successfully utilized for the preparation of alkenes from alcohols. However, it went into oblivion for nearly a decade soon after its discovery by E. M. Burgess in ...
- Sacramento - California State University
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
chm121 tutorial kit - Covenant University
... An impure compound must satisfy the following conditions during steam distillation except a. It should not decompose at the steam temperature (b) It should have a fairly high vapour present at 373K (c) It should be insoluble in water (d) The impurities present should be volatile Calculate the distan ...
... An impure compound must satisfy the following conditions during steam distillation except a. It should not decompose at the steam temperature (b) It should have a fairly high vapour present at 373K (c) It should be insoluble in water (d) The impurities present should be volatile Calculate the distan ...
74 CHAPTER-IV "LEAD (IV) ACETATE OXIDATIONS"
... have been made in recent years to direct these oxidations towards products formed through electrophilic oxyplumbation by employing more electrophilic Lead (IV) salts. Lead (IV) acetate appears to have been first used as an oxidant by Dimroth, Friedamann, Kamerer. ...
... have been made in recent years to direct these oxidations towards products formed through electrophilic oxyplumbation by employing more electrophilic Lead (IV) salts. Lead (IV) acetate appears to have been first used as an oxidant by Dimroth, Friedamann, Kamerer. ...
Aldehydes and ketones
... aromatic). The other bond to the carbonyl is either to a Hatom or another carbon group: General formula for an ester: ...
... aromatic). The other bond to the carbonyl is either to a Hatom or another carbon group: General formula for an ester: ...
AMINES
... Q20) Amines are less acidic than alcohols of comparable molecular masses Ans) Due lower electro-negativity of N compared to oxygen N-H bond is less polar than O-H bond. Q21) Gabriel phthalimide synthesis is preferred for synthesizing only primary amines. Ans) This method yields only primary amines a ...
... Q20) Amines are less acidic than alcohols of comparable molecular masses Ans) Due lower electro-negativity of N compared to oxygen N-H bond is less polar than O-H bond. Q21) Gabriel phthalimide synthesis is preferred for synthesizing only primary amines. Ans) This method yields only primary amines a ...
Chapter 15
... more electronegative (2.20 v. 2.04) and so the boron has a partial plus charge and the hydrogen has a partial negative charge. The addition occurs in one step in a concerted fashion so we see syn addition. Then the boron is replaced stereospecifically to give the less substituted alcohol. (3) Hydrol ...
... more electronegative (2.20 v. 2.04) and so the boron has a partial plus charge and the hydrogen has a partial negative charge. The addition occurs in one step in a concerted fashion so we see syn addition. Then the boron is replaced stereospecifically to give the less substituted alcohol. (3) Hydrol ...
Organic Chemistry Fifth Edition
... Steric factors seem to control the regioselectivity. The transition state that leads to 1-butene is less crowded than the one leading to cis or trans-2-butene. ...
... Steric factors seem to control the regioselectivity. The transition state that leads to 1-butene is less crowded than the one leading to cis or trans-2-butene. ...
Oxidation of Diols and Ethers by NaBr03
... molar amounts of NaBr03/NaHS03, 2-bromo-l,3-cyclohexanedione 22 was obtained in preference to the expected 1,3cyclohexanedione (40) (Table 2, Run 2). A plausible reaction path for the production of 22 from 19 is shown in Scheme 1. In a previous paper, we showed that enones upon treatment with NaBr03 ...
... molar amounts of NaBr03/NaHS03, 2-bromo-l,3-cyclohexanedione 22 was obtained in preference to the expected 1,3cyclohexanedione (40) (Table 2, Run 2). A plausible reaction path for the production of 22 from 19 is shown in Scheme 1. In a previous paper, we showed that enones upon treatment with NaBr03 ...
No Slide Title
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
The alcohols
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
aryl halides
... carbon is sp3 hybridized. Hence it is more difficult to break this bond and aryl halides resist the typical nucleophilic substitution reactions of alkyl halides. ...
... carbon is sp3 hybridized. Hence it is more difficult to break this bond and aryl halides resist the typical nucleophilic substitution reactions of alkyl halides. ...
Amines
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
Isomeric Product Detection in the
... reacted per OH-particle collision, are found to be larger than one for these reactions, providing clear evidence for particlephase secondary chain chemistry. These secondary chain reactions may in turn alter the distribution of functionalization and fragmentation particle phase products. Furthermore ...
... reacted per OH-particle collision, are found to be larger than one for these reactions, providing clear evidence for particlephase secondary chain chemistry. These secondary chain reactions may in turn alter the distribution of functionalization and fragmentation particle phase products. Furthermore ...
Ch 7 - Practice problem (Answers)
... 34. Zaitsev's rule can be used to predict the major product for which of the following reactions? A) 2-methylpentane + Br2(with light) B) 2-bromo-2-methylpentane + NaOCH2CH3 (in ethanol) C) 2-methyl-2-pentanol + PBr3 D) 2-methyl-2-pentanol + HCl Ans: B 35. The acid-catalyzed dehydration of the alcoh ...
... 34. Zaitsev's rule can be used to predict the major product for which of the following reactions? A) 2-methylpentane + Br2(with light) B) 2-bromo-2-methylpentane + NaOCH2CH3 (in ethanol) C) 2-methyl-2-pentanol + PBr3 D) 2-methyl-2-pentanol + HCl Ans: B 35. The acid-catalyzed dehydration of the alcoh ...
Exam #3
... A) Using mechanistic reasoning, EXPLAIN WHY menthyl chloride gives only 2-menthene as its E2 elimination product. B) Explain why 3-menthene (rather than 2- menthene) is the major product in the E2 elimination of neomenthyl chloride. H H3C ...
... A) Using mechanistic reasoning, EXPLAIN WHY menthyl chloride gives only 2-menthene as its E2 elimination product. B) Explain why 3-menthene (rather than 2- menthene) is the major product in the E2 elimination of neomenthyl chloride. H H3C ...
phenols - Gneet`s
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
Chemistry Unit 1
... carbonates, hydrogen carbonates, cyanides and cyanates. Besides carbon, these compounds contain a few other elements such as hydrogen, oxygen, nitrogen, sulphur, halogens and phosphorus. The branch of chemistry that studies carbon compounds is called organic chemistry. This branch of chemistry was d ...
... carbonates, hydrogen carbonates, cyanides and cyanates. Besides carbon, these compounds contain a few other elements such as hydrogen, oxygen, nitrogen, sulphur, halogens and phosphorus. The branch of chemistry that studies carbon compounds is called organic chemistry. This branch of chemistry was d ...
Tiffeneau–Demjanov rearrangement

The Tiffeneau–Demjanov rearrangement (TDR) is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone.The Tiffeneau–Demjanov ring expansion, Tiffeneau–Demjanov rearrangement, or TDR, provides an easy way to increase amino-substituted cycloalkanes and cycloalkanols in size by one carbon. Ring sizes from cyclopropane through cyclooctane are able to undergo Tiffeneau–Demjanov ring expansion with some degree of success. Yields decrease as initial ring size increases, and the ideal use of TDR is for synthesis of five, six, and seven membered rings. A principal synthetic application of Tiffeneau–Demjanov ring expansion is to bicyclic or polycyclic systems. Several reviews on this reaction have been published.