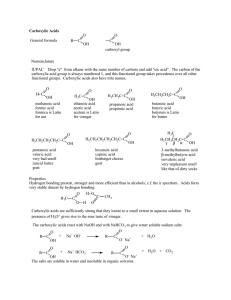
Carboxylic Acids General formula R C O OH C O OH carboxyl group
... very unpleasant smell like that of dirty socks ...
... very unpleasant smell like that of dirty socks ...
C3.5 - The John Warner School
... In the first activity in the Student Book (‘Raising the cost of alcoholic drinks?’) encourage students to think about how they could discover reliable data about the positive and negative effects of the cost of alcohol and the effect on society. Research the move to increase the price of alcoholic ...
... In the first activity in the Student Book (‘Raising the cost of alcoholic drinks?’) encourage students to think about how they could discover reliable data about the positive and negative effects of the cost of alcohol and the effect on society. Research the move to increase the price of alcoholic ...
Exam 2
... worth studying. Also problems assigned for the text may also be helpful. Chap 9 &10-- Sn2, Sn1, E2 and E1 reactions. -Know the definitions of Sn2, Sn1, E2 and E1. -Be able to depict the reaction coordinate diagrams of each reaction -Be able to draw the mechanism, products and the stereochemical resu ...
... worth studying. Also problems assigned for the text may also be helpful. Chap 9 &10-- Sn2, Sn1, E2 and E1 reactions. -Know the definitions of Sn2, Sn1, E2 and E1. -Be able to depict the reaction coordinate diagrams of each reaction -Be able to draw the mechanism, products and the stereochemical resu ...
Functional Groups
... If the carbon is attached to one other carbon that carbon is primary (1o) and the alkyl halide is also 1o If the carbon is attached to two other carbons, that carbon is secondary (2 o) and the alkyl halide is 2o If the carbon is attached to three other carbons, the carbon is tertiary (3 o) and the a ...
... If the carbon is attached to one other carbon that carbon is primary (1o) and the alkyl halide is also 1o If the carbon is attached to two other carbons, that carbon is secondary (2 o) and the alkyl halide is 2o If the carbon is attached to three other carbons, the carbon is tertiary (3 o) and the a ...
Carbonyl Classification Tests The samples to be tested in each of
... Shake the contents of the tube. A positive test is indicated by the disappearance of the orange color of the chromic acid reagent and the formation of a green or blue-green precipitate or emulsion. Primary and secondary alcohols and aliphatic aldehydes give a positive test within 5 minutes, while ar ...
... Shake the contents of the tube. A positive test is indicated by the disappearance of the orange color of the chromic acid reagent and the formation of a green or blue-green precipitate or emulsion. Primary and secondary alcohols and aliphatic aldehydes give a positive test within 5 minutes, while ar ...
Chapter 11: Reactions at an sp3 Hybridized Carbon III
... • Normally only HBr, HI, and H2SO4 are acidic enough to cleave ethers • In this case, however, the stability of tertiary carbocation which results from H– shifting and substituting for CH3OH makes this reaction work with HCl • If tertiary carbocations can be formed then HCl is strong enough ...
... • Normally only HBr, HI, and H2SO4 are acidic enough to cleave ethers • In this case, however, the stability of tertiary carbocation which results from H– shifting and substituting for CH3OH makes this reaction work with HCl • If tertiary carbocations can be formed then HCl is strong enough ...
Curriculum Project
... 2. The more bonds the stronger the bond and the carbon atoms loose the ability to rotate. 3. Acetylene, C2H2, has a triple bond between the carbon atoms and methane has no carbon-carbon bond so there is much more energy that can be released. 4. Look at the structural differences between single and d ...
... 2. The more bonds the stronger the bond and the carbon atoms loose the ability to rotate. 3. Acetylene, C2H2, has a triple bond between the carbon atoms and methane has no carbon-carbon bond so there is much more energy that can be released. 4. Look at the structural differences between single and d ...
1-14 Amines Amides
... Again, the nitrogen atom is attached to the number one carbon, so part of the name is 1-aminoethane. Since there are two methyl groups attached to the nitrogen, we add N,N-dimethyl- to the front of the name. Note each methyl group gets its own locator, thus there are two Ns in the name. The IUPAC na ...
... Again, the nitrogen atom is attached to the number one carbon, so part of the name is 1-aminoethane. Since there are two methyl groups attached to the nitrogen, we add N,N-dimethyl- to the front of the name. Note each methyl group gets its own locator, thus there are two Ns in the name. The IUPAC na ...
Organic Chemistry II Introduction
... ArOH is more acidic than ROH Soluble in dilute NaOH Anion is resonance stabilized EWG make phenols more acidic than phenol EDG make phenols less acidic than phenol O ...
... ArOH is more acidic than ROH Soluble in dilute NaOH Anion is resonance stabilized EWG make phenols more acidic than phenol EDG make phenols less acidic than phenol O ...
Assignment Sheet
... Explain how structure and bonding of carbon lead to the diversity and number of organic compounds. Compare structural and geometric isomers of organic compounds. Distinguish among the structures of alkanes, alkenes, alkynes, and aromatic hydrocarbons. Write structural formulas and names for alkanes, ...
... Explain how structure and bonding of carbon lead to the diversity and number of organic compounds. Compare structural and geometric isomers of organic compounds. Distinguish among the structures of alkanes, alkenes, alkynes, and aromatic hydrocarbons. Write structural formulas and names for alkanes, ...
Ketones - Sanfordchemistrystudentwork
... on the carbonyl group determines ketones from alcohols and ethers. A carbon atom across a carbonyl group is often referred to as an a-carbon and the hydrogen atoms connected to the center of an a-carbon are called a-hydrogen. Ketones with a-hydrogen centers experience a Keto-enol tautomerism (a chem ...
... on the carbonyl group determines ketones from alcohols and ethers. A carbon atom across a carbonyl group is often referred to as an a-carbon and the hydrogen atoms connected to the center of an a-carbon are called a-hydrogen. Ketones with a-hydrogen centers experience a Keto-enol tautomerism (a chem ...
Class Notes Test 1
... • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and handling: Grignard reactants RMgBr must be made, stored, and handled in special ...
... • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and handling: Grignard reactants RMgBr must be made, stored, and handled in special ...
Mr. Kent`s Organic Chemistry Unit Notes B. ______ will not only
... hydrocarbons (hydrogen and carbon only) containing 1 carbon to carbon triple bond "C≡C". The rule for naming is they all end with "-yne". The general formula is CnH2n-2, n is the number of carbons is used to determine the number of hydrogen atoms. Example n=5, so H=(2(5)-2)=8 Rule #1-Name the longes ...
... hydrocarbons (hydrogen and carbon only) containing 1 carbon to carbon triple bond "C≡C". The rule for naming is they all end with "-yne". The general formula is CnH2n-2, n is the number of carbons is used to determine the number of hydrogen atoms. Example n=5, so H=(2(5)-2)=8 Rule #1-Name the longes ...
Nucleophilic Substitution Reaction
... carbon; these two carbon atoms are usually referred to as - and -carbons, respectively. ...
... carbon; these two carbon atoms are usually referred to as - and -carbons, respectively. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.























