16.2: Structure and Bonding in Ethers and Epoxides
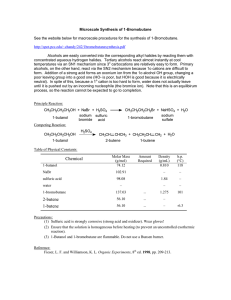
... Reaction of an alkoxide with an alkyl halide or tosylate to give an ether. Alkoxides are prepared by the reaction of an alcohol with a strong base such as sodium hydride (NaH) ...
... Reaction of an alkoxide with an alkyl halide or tosylate to give an ether. Alkoxides are prepared by the reaction of an alcohol with a strong base such as sodium hydride (NaH) ...
Functional Group Handout
... E. Amides: contain a carbonyl group. The carbon atom of the carbonyl group is bonded to another carbon atom and a nitrogen atom. Amides can be primary secondary or tertiary. The nitrogen atom of the primary amide is bonded to the carbonyl carbon and two hydrogens. The nitrogen atom of a secondary am ...
... E. Amides: contain a carbonyl group. The carbon atom of the carbonyl group is bonded to another carbon atom and a nitrogen atom. Amides can be primary secondary or tertiary. The nitrogen atom of the primary amide is bonded to the carbonyl carbon and two hydrogens. The nitrogen atom of a secondary am ...
Synthesis of 1
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
... http://spot.pcc.edu/~chandy/242/1bromobutanesynthesis.pdf Alcohols are easily converted into the corresponding alkyl halides by reacting them with concentrated aqueous hydrogen halides. Tertiary alcohols react almost instantly at cool temperatures via an SN1 mechanism since 3o carbocations are relat ...
ORGANIC CHEMISTRY
... • Carbon compounds are considered organic EXCEPT for – carbon dioxide, – carbides, and – carbonates. ...
... • Carbon compounds are considered organic EXCEPT for – carbon dioxide, – carbides, and – carbonates. ...
Final Study Guide
... The final exam, on Tuesday June 12th, is a comprehensive exam. It is open to lecture and lab notes but closed to chemistry textbooks. You do not need to rely on memorization as much but have the ability to solve the problems and use provided information. Quizzes, homework problems, practice exams, e ...
... The final exam, on Tuesday June 12th, is a comprehensive exam. It is open to lecture and lab notes but closed to chemistry textbooks. You do not need to rely on memorization as much but have the ability to solve the problems and use provided information. Quizzes, homework problems, practice exams, e ...
Unit 13: Organic Chemistry
... • –OH is called a hydroxyl group (not to be confused with OH-!) • When a hydroxyl group is attached to an organic molecule it is called an alcohol • Alcohols are named by adding –ol to the parent chain and using a number to indicate the location ...
... • –OH is called a hydroxyl group (not to be confused with OH-!) • When a hydroxyl group is attached to an organic molecule it is called an alcohol • Alcohols are named by adding –ol to the parent chain and using a number to indicate the location ...
Organometallic Compounds: Alkyllithium Reagent
... Alcohols from Carbonyls and Grignard Reagents Esters react with two molecules of Grignard reagents to form tert-alcohols ...
... Alcohols from Carbonyls and Grignard Reagents Esters react with two molecules of Grignard reagents to form tert-alcohols ...
Suggest a reason for the large difference in the boiling points of
... 2) reaction to distinguish between 1 , 2 and 3 alcohols 12 An aqueous solution of a gas (X) shows following reactions: It turns red litmus blue.When added in excess to a copper sulphate solution, a deep blue colour is obtained.On addition of ferric chloride solution a brownish precipitate soluble in ...
... 2) reaction to distinguish between 1 , 2 and 3 alcohols 12 An aqueous solution of a gas (X) shows following reactions: It turns red litmus blue.When added in excess to a copper sulphate solution, a deep blue colour is obtained.On addition of ferric chloride solution a brownish precipitate soluble in ...
Chapter 10 - UCSB CLAS
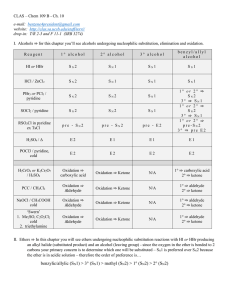
... undergoing Hoffmann (aka anti-Zaitsev) elimination reactions (only E2)– there are four carbons bonded to the nitrogen and therefore there can be up to twelve β-carbons to choose from – however in an anti-Zaitsev elimination the order of preference is benzylic/allylic > 1° > 2° > 3° VII. Sulfur funct ...
... undergoing Hoffmann (aka anti-Zaitsev) elimination reactions (only E2)– there are four carbons bonded to the nitrogen and therefore there can be up to twelve β-carbons to choose from – however in an anti-Zaitsev elimination the order of preference is benzylic/allylic > 1° > 2° > 3° VII. Sulfur funct ...
Functional Group Naming Rules
... The 2 in 2-propanone is redundant since it can have only one configuration. However, in naming ketones the number on the carbon chain is given even if only one possibility exists. ...
... The 2 in 2-propanone is redundant since it can have only one configuration. However, in naming ketones the number on the carbon chain is given even if only one possibility exists. ...
Hydroformylation Hydroformylation, also known as oxo synthesis or
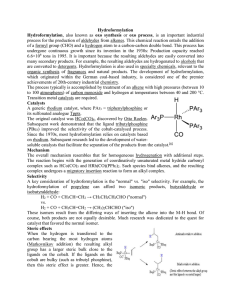
... Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous ...
... Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous ...
Lab Evaporation and Intermolecular Attraction
... alkanes are pentane, C5H12, and hexane, C6H14. In addition to carbon and hydrogen atoms, alcohols also contain the -OH functional group. Methanol, CH3OH, and ethanol, C2H5OH, are two of the alcohols that we will use in this experiment. You will examine the molecular structure of alkanes and alcohols ...
... alkanes are pentane, C5H12, and hexane, C6H14. In addition to carbon and hydrogen atoms, alcohols also contain the -OH functional group. Methanol, CH3OH, and ethanol, C2H5OH, are two of the alcohols that we will use in this experiment. You will examine the molecular structure of alkanes and alcohols ...
Yeast Reduction #812
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.