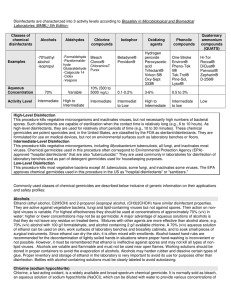
click - Chemsheets
... • Acidified potassium dichromate, contains Cr2O72• Used to test for alcohols (1y and 2y) & aldehydes – goes from orange Cr2O72- to green Cr3+ • Reduced from Cr(+6) to Cr(+3) ...
... • Acidified potassium dichromate, contains Cr2O72• Used to test for alcohols (1y and 2y) & aldehydes – goes from orange Cr2O72- to green Cr3+ • Reduced from Cr(+6) to Cr(+3) ...
Proximate Analysis
... this can certainly be taken for granted with the overwhelmingly greater part of the triglycerides of fat-rich tissues. (The same is probably true for waxes.) Moreover, triglycerides are easily soluble in practically all fat solvents, an exception being cold alcohol. Thus the wet tissue may be extrac ...
... this can certainly be taken for granted with the overwhelmingly greater part of the triglycerides of fat-rich tissues. (The same is probably true for waxes.) Moreover, triglycerides are easily soluble in practically all fat solvents, an exception being cold alcohol. Thus the wet tissue may be extrac ...
Name: Date: Page 1 of 3 Organic Alcohols You are researching the
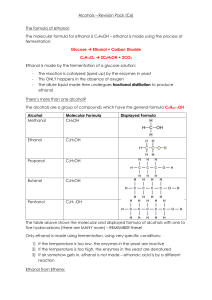
... Chemical formulas are used to represent the formulas for alcohol molecules. For example, ethanol can be represented as . In this formula, represents the five hydrogen atoms that are connected to the two carbon atoms. If C represents the number of carbon atoms in an organic alcohol molecule, then the ...
... Chemical formulas are used to represent the formulas for alcohol molecules. For example, ethanol can be represented as . In this formula, represents the five hydrogen atoms that are connected to the two carbon atoms. If C represents the number of carbon atoms in an organic alcohol molecule, then the ...
Activity 1.1.3 Organic Alcohols
... 10. See if you can figure out a formula that will work for any organic alcohol. Going from a pattern to a formula is a guess and check process. You have some practice guessing, so give it a try! ...
... 10. See if you can figure out a formula that will work for any organic alcohol. Going from a pattern to a formula is a guess and check process. You have some practice guessing, so give it a try! ...
aminoalkanes (or amines)
... AMINOALKANES (OR AMINES) Introduction: The IUPAC names for amines are aminoalkanes or alkanamines. E.g.: CH3CH2NH2 Ethylamine – amine –common name Aminoethane – aminoalkane – systematic name Ethanamine – alkanamine – systematic name Mines all contain a nitrogen atom in the organic molecule. Th ...
... AMINOALKANES (OR AMINES) Introduction: The IUPAC names for amines are aminoalkanes or alkanamines. E.g.: CH3CH2NH2 Ethylamine – amine –common name Aminoethane – aminoalkane – systematic name Ethanamine – alkanamine – systematic name Mines all contain a nitrogen atom in the organic molecule. Th ...
Alcohol oxidation
... Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates com ...
... Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates com ...
1.7 FUNCTIONAL GROUPS
... Certain combinations of bonds show up repeatedly in organic chemistry and organic chemists give those bonding combinations specific names. It is very useful to know the names of those specific types of bonds. Examples are shown below and you should make flash cards and learn them by heart. There can ...
... Certain combinations of bonds show up repeatedly in organic chemistry and organic chemists give those bonding combinations specific names. It is very useful to know the names of those specific types of bonds. Examples are shown below and you should make flash cards and learn them by heart. There can ...
Sources of hydride ion
... Reactions wiith water Water adds to an aldehyde or kettone to form a hydrate: ...
... Reactions wiith water Water adds to an aldehyde or kettone to form a hydrate: ...
Organic Chemistry
... The OH group is polar; hydrogen bonding makes alcohols more soluble in water than in hydrocarbons CH3OH methyl alcohol, methanol, “wood alcohol”. Impurity in moonshine; causes blindness CH3CH2OH ethyl alcohol, ethanol, “alcohol” Prepared by fermentation of sugarcontaining plant material. ...
... The OH group is polar; hydrogen bonding makes alcohols more soluble in water than in hydrocarbons CH3OH methyl alcohol, methanol, “wood alcohol”. Impurity in moonshine; causes blindness CH3CH2OH ethyl alcohol, ethanol, “alcohol” Prepared by fermentation of sugarcontaining plant material. ...
Organic Chemistry
... CH3OH methyl alcohol, methanol, “wood alcohol”. Impurity in moonshine; causes blindness CH3CH2OH ethyl alcohol, ethanol, “alcohol” Prepared by fermentation of sugar-containing plant material. ...
... CH3OH methyl alcohol, methanol, “wood alcohol”. Impurity in moonshine; causes blindness CH3CH2OH ethyl alcohol, ethanol, “alcohol” Prepared by fermentation of sugar-containing plant material. ...
selection of a disinfectant
... considerably reduced by organic matter (protein). Storage of stock or working solutions of bleach in open containers, particularly at high temperatures, releases chlorine gas thus weakening their germicidal potential. The frequency with which working solutions of bleach should be changed depends on ...
... considerably reduced by organic matter (protein). Storage of stock or working solutions of bleach in open containers, particularly at high temperatures, releases chlorine gas thus weakening their germicidal potential. The frequency with which working solutions of bleach should be changed depends on ...
level three chemistry: organics
... I can show that I understand the significance of the structure of each functional group by explaining the relative solubility of each functional group in terms of polarity and hydrogen bonding. I can show that I understand the significance of the structure of each functional group by explaining the ...
... I can show that I understand the significance of the structure of each functional group by explaining the relative solubility of each functional group in terms of polarity and hydrogen bonding. I can show that I understand the significance of the structure of each functional group by explaining the ...
... we use microwave method [11-15]. They provide an efficient and useful synthetic method of diverse and complex compounds, as well as small and drug-like heterocycles [16-19]. In a higher product yield than classical chemistry [20-25]. The importance of these processes is underscored by the large numb ...
Chapter 18 - Hope Charter School
... b. Naming alkynes 1) similar to alkenes, but with the suffix –yne 2) make sure that the triple bond gets the lowest number possible c. Properties ...
... b. Naming alkynes 1) similar to alkenes, but with the suffix –yne 2) make sure that the triple bond gets the lowest number possible c. Properties ...
Organic Compounds Containing C, H and O
... 1. Arrange the following in the increasing order of their acid strength. Benzoic acid, 4-nitrobenzoic acid, 3, 4-dinitro benzoic acid, 4-methoxy benzoic acid. Ans. Increasing order of acidic strength is: 4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3, 4-dinitrobenzoic acid. reason: ...
... 1. Arrange the following in the increasing order of their acid strength. Benzoic acid, 4-nitrobenzoic acid, 3, 4-dinitro benzoic acid, 4-methoxy benzoic acid. Ans. Increasing order of acidic strength is: 4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3, 4-dinitrobenzoic acid. reason: ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.