Derivatization reactions for the determination of amines by gas
... Phosphoamide formation Dimethylthiophosphinicchloride Dimethylthiophosphorylchloride Diethylthiophosphoryl chloride ...
... Phosphoamide formation Dimethylthiophosphinicchloride Dimethylthiophosphorylchloride Diethylthiophosphoryl chloride ...
Alkenes 4 - ChemWeb (UCC)
... Long before anything was known about the mechanism of this reaction it was recognised that 'Addition of HX to an alkene will proceed in such a way as to attach hydrogen to the least substituted carbon and X to the most substituted carbon'. This is known as Markovnikov's Rule after the Russian chemis ...
... Long before anything was known about the mechanism of this reaction it was recognised that 'Addition of HX to an alkene will proceed in such a way as to attach hydrogen to the least substituted carbon and X to the most substituted carbon'. This is known as Markovnikov's Rule after the Russian chemis ...
Chiral Enolate Equivalents
... Despite this impressive track record, the weak reactivity of enolates in general confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophile ...
... Despite this impressive track record, the weak reactivity of enolates in general confines the range of usable electrophiles to aldehydes, some primary or activated alkyl halides, unsaturated carbonyls, electrophilic halogens, oxaziridines, aza compounds, and a handful of other reactive electrophile ...
Chapter 14 Aldehydes, Ketones, and Chiral Molecules
... Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
... Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
Aldehydes, Ketones, & Chiral Molecules
... attached to at least one H atom. In a ketone is attached to two carbon groups. ...
... attached to at least one H atom. In a ketone is attached to two carbon groups. ...
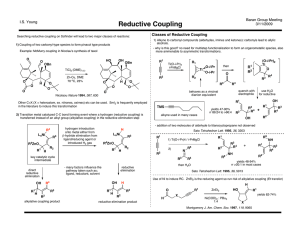
Reductive Couplings
... - desired reaction is three-component alkylative coupling (RC was a competing problem) - imines less electrophilic - need hydroxylic solvent and organoboron reagent - methanol occupies coordination site, hindering β-hydride elimination ...
... - desired reaction is three-component alkylative coupling (RC was a competing problem) - imines less electrophilic - need hydroxylic solvent and organoboron reagent - methanol occupies coordination site, hindering β-hydride elimination ...
an introduction to organic reactions
... spending time working through the problem sets and going to your invaluable TA's at section, then you'll probably find orgo to be a challenging class but not unreasonably so. ...
... spending time working through the problem sets and going to your invaluable TA's at section, then you'll probably find orgo to be a challenging class but not unreasonably so. ...
doc
... Structural Isomers are compounds with the same chemical formula but different arrangement of atoms. For example, the ortho, meta and para forms of dichlorobenzene shown above are all isomers with the chemical formula C6H4Cl2. These compounds have the same molecular mass, but their physical and chemi ...
... Structural Isomers are compounds with the same chemical formula but different arrangement of atoms. For example, the ortho, meta and para forms of dichlorobenzene shown above are all isomers with the chemical formula C6H4Cl2. These compounds have the same molecular mass, but their physical and chemi ...
Alcohols, Ethers, and Epoxides
... The structure and properties of three simple alcohols—methanol, 2-propanol, and ethylene glycol—are given in Figure 9.3. Ethanol (CH3CH2OH), formed by the fermentation of the carbohydrates in grains, grapes, and potatoes, is the alcohol present in alcoholic beverages. It is perhaps the first organic ...
... The structure and properties of three simple alcohols—methanol, 2-propanol, and ethylene glycol—are given in Figure 9.3. Ethanol (CH3CH2OH), formed by the fermentation of the carbohydrates in grains, grapes, and potatoes, is the alcohol present in alcoholic beverages. It is perhaps the first organic ...
irm_ch14
... 14.83 There is no hydrogen bonding between molecules of dimethyl ether (disruptive forces are greater than cohesive forces); there is hydrogen bonding between molecules of ethyl alcohol (cohesive forces are of about the same magnitude as disruptive forces). 14.84 Alcohols are more soluble because of ...
... 14.83 There is no hydrogen bonding between molecules of dimethyl ether (disruptive forces are greater than cohesive forces); there is hydrogen bonding between molecules of ethyl alcohol (cohesive forces are of about the same magnitude as disruptive forces). 14.84 Alcohols are more soluble because of ...
Haloalkanes-haloarenes
... CH3–CH=CH2 to CH3–CH2–CH2–OH A. CH3–CH2=CH2 + HBr org. peroxide CH3–CH2–CH2Br ii) Ethanol to butyne–1 CH3–CH2OH A. CH3CH2OH HBr ...
... CH3–CH=CH2 to CH3–CH2–CH2–OH A. CH3–CH2=CH2 + HBr org. peroxide CH3–CH2–CH2Br ii) Ethanol to butyne–1 CH3–CH2OH A. CH3CH2OH HBr ...
ch12 - Organic Compounds with Oxygen and Sulfur
... B. CH3OCH2CH3 Slightly soluble Ethers with less than four carbons are only slightly soluble in water. C. CH3CH2OH Soluble Short-chain alcohols form hydrogen bonds with water. ...
... B. CH3OCH2CH3 Slightly soluble Ethers with less than four carbons are only slightly soluble in water. C. CH3CH2OH Soluble Short-chain alcohols form hydrogen bonds with water. ...
Reactions of Alkenes
... The only reaction covered so far for preparing alkanes is catalytic hydrogenation of alkenes. This leads to a new question. "Starting with anything, how can I prepare cyclohexene in a single step by a reaction I am sure will work?" ...
... The only reaction covered so far for preparing alkanes is catalytic hydrogenation of alkenes. This leads to a new question. "Starting with anything, how can I prepare cyclohexene in a single step by a reaction I am sure will work?" ...
Chem. Soc. Rev., 2015, 44, 2202--2220 - RSC Publishing
... of the Si–H bond. The focus of this section is on hydrosilylation of CQX bonds, initially introduced by Piers and co-workers.3,5 The ground-breaking discovery by Stephan and, independently, Klankermayer that the same catalyst also activates the H–H bond led to the development of several related hydr ...
... of the Si–H bond. The focus of this section is on hydrosilylation of CQX bonds, initially introduced by Piers and co-workers.3,5 The ground-breaking discovery by Stephan and, independently, Klankermayer that the same catalyst also activates the H–H bond led to the development of several related hydr ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.