Sect 5 NMR Trends
... so alkenes and aromatics (and other p-bonds) are not isotropic – they have effects that are different in different directions – we call them ANISOTROPIC Circulation of p the -electrons around the ring ...
... so alkenes and aromatics (and other p-bonds) are not isotropic – they have effects that are different in different directions – we call them ANISOTROPIC Circulation of p the -electrons around the ring ...
Diastereoselective Allylation of Carbonyl Compounds and Imines:
... through the conformation where destabilizing gauche interactions are minimized. In contrast, for Mg, Ti, B, and In allylic derivatives, a cyclic six-membered Zimmerman−Traxler7 type transition state is usually invoked. In the cyclic model, it is generally proposed that aldehydes locate the H (R2 = H ...
... through the conformation where destabilizing gauche interactions are minimized. In contrast, for Mg, Ti, B, and In allylic derivatives, a cyclic six-membered Zimmerman−Traxler7 type transition state is usually invoked. In the cyclic model, it is generally proposed that aldehydes locate the H (R2 = H ...
A. 3-chloro-1-ethoxybutane B. p
... a. Hydrogen bonding between like molecules (aldehyde with aldehyde) b. Hydrogen bonding with water c. Dipolar bonding between like molecules (aldehyde with aldehyde) d. Both b and c e. Both a and c ANS: D ...
... a. Hydrogen bonding between like molecules (aldehyde with aldehyde) b. Hydrogen bonding with water c. Dipolar bonding between like molecules (aldehyde with aldehyde) d. Both b and c e. Both a and c ANS: D ...
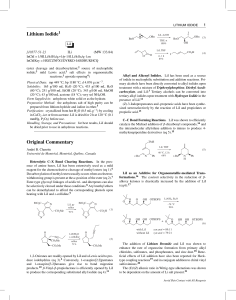
Lorell Thesis Final Version in PDF S
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
chapter 22 organic and biological molecules
... functional group. The monomer for a homopolymer polyester must have an alcohol functional group and a carboxylic acid functional group present in the structure. b. A polyamide forms when an amine functional group reacts with a carboxylic acid functional group. For a copolymer polyamide, one monomer ...
... functional group. The monomer for a homopolymer polyester must have an alcohol functional group and a carboxylic acid functional group present in the structure. b. A polyamide forms when an amine functional group reacts with a carboxylic acid functional group. For a copolymer polyamide, one monomer ...
Nucleophilic Acyl Substitution
... compound responsible for the burning sensation felt in muscles during anaerobic exercise, and it is also found in sour milk. Spinach and other leafy green vegetables are rich in oxalic acid. Succinic acid and citric acid are important intermediates in the citric acid cycle (Section 25.1), a series o ...
... compound responsible for the burning sensation felt in muscles during anaerobic exercise, and it is also found in sour milk. Spinach and other leafy green vegetables are rich in oxalic acid. Succinic acid and citric acid are important intermediates in the citric acid cycle (Section 25.1), a series o ...
2009 Nov (9746) Paper 1
... When Cu2+(aq) and I–(aq) are mixed, the brown precipitate formed is Cu2I2(s) in brown I2(aq). On adding S2O32–(aq), the brown colour disappears which suggests that I2 is reduced to I–. The white precipitate that remains is Cu2I2. o o ...
... When Cu2+(aq) and I–(aq) are mixed, the brown precipitate formed is Cu2I2(s) in brown I2(aq). On adding S2O32–(aq), the brown colour disappears which suggests that I2 is reduced to I–. The white precipitate that remains is Cu2I2. o o ...
Document
... acids (R-COOH), aldehydes (R-COH) – what are the others presented in the book? • Identifying these! • Naming these (all have their own endings)! ...
... acids (R-COOH), aldehydes (R-COH) – what are the others presented in the book? • Identifying these! • Naming these (all have their own endings)! ...
Document
... acids (R-COOH), aldehydes (R-COH) – what are the others presented in the book? • Identifying these! • Naming these (all have their own endings)! ...
... acids (R-COOH), aldehydes (R-COH) – what are the others presented in the book? • Identifying these! • Naming these (all have their own endings)! ...
lecture 12 catalysis_transformation of alkenes_alkynes
... SAMPLE PROBLEM: Assume 2-pentene and 2-hexene undergo metathesis. AT equilibrium what are all the possible alkenes that would be present, neglecting stereochemistry about the double bond? Remember to consider self metathesis reactions. ...
... SAMPLE PROBLEM: Assume 2-pentene and 2-hexene undergo metathesis. AT equilibrium what are all the possible alkenes that would be present, neglecting stereochemistry about the double bond? Remember to consider self metathesis reactions. ...
CHAPTER 21 ORGANIC CHEMISTRY
... The R designation refers to the rest of the organic molecule beyond the specific functional group indicated in the formula. The R group may be a hydrogen but is usually a hydrocarbon fragment. The major point in the R group designation is that if the R group is an organic fragment, then the first at ...
... The R designation refers to the rest of the organic molecule beyond the specific functional group indicated in the formula. The R group may be a hydrogen but is usually a hydrocarbon fragment. The major point in the R group designation is that if the R group is an organic fragment, then the first at ...
N - Clayton State University
... Alcohols contain an OH group connected to a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ring Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities Eth ...
... Alcohols contain an OH group connected to a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ring Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities Eth ...
chapter2
... conformation: a conformation about a carbon-carbon single bond in which the atoms or groups of atoms on one carbon are as close as possible to the atoms or groups of atoms on an adjacent carbon H ...
... conformation: a conformation about a carbon-carbon single bond in which the atoms or groups of atoms on one carbon are as close as possible to the atoms or groups of atoms on an adjacent carbon H ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.












![[Ru(Triphos)H2(CO)] Characterisation - Durham e](http://s1.studyres.com/store/data/017676948_1-4352644236c53cc416f065328f560d26-300x300.png)