Dr Davids Essential Chemistry Definitions Bk1
... Chemistry definitions are statements of the precise meanings of chemical terms. A GCE examination question may start by asking for a particular definition. Usually they carry two marks and in order to achieve full marks the definition must be correct in all respects. It is well worth while learning ...
... Chemistry definitions are statements of the precise meanings of chemical terms. A GCE examination question may start by asking for a particular definition. Usually they carry two marks and in order to achieve full marks the definition must be correct in all respects. It is well worth while learning ...
Organic Reactions Summary
... Adding groups (or atoms) to a chain by breaking a C=C bond Family Alkenes Alkenes ...
... Adding groups (or atoms) to a chain by breaking a C=C bond Family Alkenes Alkenes ...
Organic Chemistry Review
... Meth = 1 C Eth = 2 C Prop = 3 C But = 4 C Pent = 5 C Hex = 6 C Hept = 7 C Oct = 8 C Non = 9 C Dec = 10 C ...
... Meth = 1 C Eth = 2 C Prop = 3 C But = 4 C Pent = 5 C Hex = 6 C Hept = 7 C Oct = 8 C Non = 9 C Dec = 10 C ...
Elimination reactions under acidic conditions
... Cumulative problems You have learned three types of reactions: acid/base, addition, and elimination. Can you think about them all at the same time? 6. Predict the products of these reactions. (Hint: What type of reaction is it?) ...
... Cumulative problems You have learned three types of reactions: acid/base, addition, and elimination. Can you think about them all at the same time? 6. Predict the products of these reactions. (Hint: What type of reaction is it?) ...
ORGANIC CHEMISTRY 03 JULY 2014 Lesson Description
... Which of these organic compounds are you most likely to smell first, if the lids of the containers were opened at the same time? ...
... Which of these organic compounds are you most likely to smell first, if the lids of the containers were opened at the same time? ...
Esters are reduced by hydride reagents to give alcohols or aldehydes.
... Long-chain carboxylic acids and phospholipids are termed amphipathic: they have hydrophobic and hydrophilic ends. When dissolved in water, fatty acids and some phospholipids form structures called “micelles.” Most phospholipids form a more complicated structure called a “lipid bilayer” when dissolv ...
... Long-chain carboxylic acids and phospholipids are termed amphipathic: they have hydrophobic and hydrophilic ends. When dissolved in water, fatty acids and some phospholipids form structures called “micelles.” Most phospholipids form a more complicated structure called a “lipid bilayer” when dissolv ...
Total Synthesis of Phorboxazole A. 2. Assembly of Subunits and
... It also reports that attempts to couple two subunits at C32C33 through addition of the anion from deprotonation of a methyl-substituted oxazole representing C20-C32 with a δ-lactone incorporating C33-C46 encountered difficulties. Specifically, this reaction resulted in a significant quantity of a te ...
... It also reports that attempts to couple two subunits at C32C33 through addition of the anion from deprotonation of a methyl-substituted oxazole representing C20-C32 with a δ-lactone incorporating C33-C46 encountered difficulties. Specifically, this reaction resulted in a significant quantity of a te ...
Chapter 5 – Chemical Reactions
... Add a catalyst – a catalyst is a chemical that speeds up a reaction but does not get used up by the reaction ...
... Add a catalyst – a catalyst is a chemical that speeds up a reaction but does not get used up by the reaction ...
C1a - Mr Corfe
... When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au silver Ag RULE: An metal is more r ...
... When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au silver Ag RULE: An metal is more r ...
Word - chemmybear.com
... Know that “cyclo-“ means the molecule contains a ring. Draw and build cyclopentane. Know that “saturated” means “saturated with hydrogens” and describes alkanes. Know that alkenes, alkynes, and cyclic hydrocarbons are all “unsaturated.” State why unsaturated fats are better for you (the doub ...
... Know that “cyclo-“ means the molecule contains a ring. Draw and build cyclopentane. Know that “saturated” means “saturated with hydrogens” and describes alkanes. Know that alkenes, alkynes, and cyclic hydrocarbons are all “unsaturated.” State why unsaturated fats are better for you (the doub ...
Lab 9 - Academic Computer Center
... and record the exact mass of the benzil in your notebook. 2. Add 5 mL of 95% ethanol to the Erlenmeyer flask that contains the benzil. 3. Cool the Erlenmeyer flask by rotating it under a stream of tap water. 4. Weigh 0.1-g NaBH4 on a waxed paper and transfer the white solid to the Erlenmeyer flask t ...
... and record the exact mass of the benzil in your notebook. 2. Add 5 mL of 95% ethanol to the Erlenmeyer flask that contains the benzil. 3. Cool the Erlenmeyer flask by rotating it under a stream of tap water. 4. Weigh 0.1-g NaBH4 on a waxed paper and transfer the white solid to the Erlenmeyer flask t ...
Lecture 15
... •Electrons are a standard currency that let us rank the reducing/oxidizing potential of different redox couples. •When the difference between the E°’ values is positive, then G° is negative because ...
... •Electrons are a standard currency that let us rank the reducing/oxidizing potential of different redox couples. •When the difference between the E°’ values is positive, then G° is negative because ...
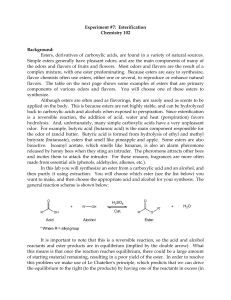
ESTERIFICATION
... so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction flask. This process allows the reaction to be heated over a period of time, without evaporating away the solvent or reactants. At the end of the reaction your product will ...
... so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction flask. This process allows the reaction to be heated over a period of time, without evaporating away the solvent or reactants. At the end of the reaction your product will ...
Amide Uses
... Amide An amide is one of three kinds of compounds: the organic functional group characterized by a carbonyl group (C=O) linked to a nitrogen atom (N), or a compound that contains this functional group (pictured to the right); or a particular kind of nitrogen anion. any organic compound derived ...
... Amide An amide is one of three kinds of compounds: the organic functional group characterized by a carbonyl group (C=O) linked to a nitrogen atom (N), or a compound that contains this functional group (pictured to the right); or a particular kind of nitrogen anion. any organic compound derived ...
Lab 7_Esterification
... It is important to note that this is a reversible reaction, so the acid and alcohol reactants and ester products are in equilibrium (implied by the double arrow). What this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in ...
... It is important to note that this is a reversible reaction, so the acid and alcohol reactants and ester products are in equilibrium (implied by the double arrow). What this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in ...
Make Your Own Summary 1. single displacement reaction 2
... neither a single reactant nor a single product, so it can be neither a synthesis reaction nor a decomposition reaction. The reactants are a compound and an element, which prevents the reaction from being a double displacement reaction. The products are two compounds, which prevent the reaction from ...
... neither a single reactant nor a single product, so it can be neither a synthesis reaction nor a decomposition reaction. The reactants are a compound and an element, which prevents the reaction from being a double displacement reaction. The products are two compounds, which prevent the reaction from ...
KINETIC AND MECHANISTIC STUDY OF OXIDATION OF ESTER
... The course of reaction was followed by measuring the absorbance (optical density) of unreacted permanganate ions from time to time at 520 nm using Carl ‐ Zeiss spectrophotometer. The reaction were followed upto 70 to 85% completion and the product were identified as acid i.e ...
... The course of reaction was followed by measuring the absorbance (optical density) of unreacted permanganate ions from time to time at 520 nm using Carl ‐ Zeiss spectrophotometer. The reaction were followed upto 70 to 85% completion and the product were identified as acid i.e ...
Ester
... You do not have to write a ‘purpose’ for this reaction—the reaction equation is considered the ‘purpose’. (you do not need to write the arrow pushing mechanism-just the reactants and products). Use the acid and alcohol that you chose in the reaction equation and clearly show the ester that they will ...
... You do not have to write a ‘purpose’ for this reaction—the reaction equation is considered the ‘purpose’. (you do not need to write the arrow pushing mechanism-just the reactants and products). Use the acid and alcohol that you chose in the reaction equation and clearly show the ester that they will ...
Day 16 – Intro to Chemistry
... A. A chemical reaction = the process by which atoms bond together or break apart. B. The reaction of two or more elements together results in the formation of a chemical bond between atoms and the formation of a chemical compound ...
... A. A chemical reaction = the process by which atoms bond together or break apart. B. The reaction of two or more elements together results in the formation of a chemical bond between atoms and the formation of a chemical compound ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.

![Reduction [H]](http://s1.studyres.com/store/data/007148356_1-25f5210a5c809c157bb6e0d32d66fd9d-300x300.png)