Chapter 20: Carboxylic Acids and Nitriles
... anion reacts with Lewis Acid AlH3, and the resulting anion complexes with Li+ in a tight ion pair; this leaves the C=N bond available for a second addition H H ...
... anion reacts with Lewis Acid AlH3, and the resulting anion complexes with Li+ in a tight ion pair; this leaves the C=N bond available for a second addition H H ...
protecting groups
... Nucleophiles interact with this orbital by doing 1,2 addition Bases interact with this orbital by deprotonation at the alpha position. ...
... Nucleophiles interact with this orbital by doing 1,2 addition Bases interact with this orbital by deprotonation at the alpha position. ...
I PUC Chemistry Mock Paper
... 11. How many atoms of gold are present in 49.25g of gold ( Atomic mass of gold = 197). 12. Define a) surface tension b) Boyle temperature. 13. What is Hydrogen bonding? Illustrate with an example. 14. How is plaster of paris prepared from gypsum? Give equation 15. Write any two differences between d ...
... 11. How many atoms of gold are present in 49.25g of gold ( Atomic mass of gold = 197). 12. Define a) surface tension b) Boyle temperature. 13. What is Hydrogen bonding? Illustrate with an example. 14. How is plaster of paris prepared from gypsum? Give equation 15. Write any two differences between d ...
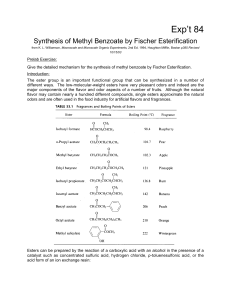
In the preparation of the esters given in this experiment
... 13. The vapor pressures of 1,2-diphenylethane, p-dichlorobenzene, and 1,3,5trichlorobenzene are 0.06, 11.2, and 1.4 torr, respectively, at their melting point (52-54 degrees C). Which compound is likely to be sublimed most rapidly at a reduced pressure of 15 torr and a temperature of 40 degrees C? 1 ...
... 13. The vapor pressures of 1,2-diphenylethane, p-dichlorobenzene, and 1,3,5trichlorobenzene are 0.06, 11.2, and 1.4 torr, respectively, at their melting point (52-54 degrees C). Which compound is likely to be sublimed most rapidly at a reduced pressure of 15 torr and a temperature of 40 degrees C? 1 ...
MS PowerPoint - Catalysis Eprints database
... I. V. Kozhevnikov, Russ. Chem. Rev. 56 (1987) 811 M. Misono, N. Mizuno, K. Katamura, A. Kasai, Y. Konishi, K. Sakata, T. Okuhara, Y. Yoneda, Bull. Chem. Soc. Jpn. 55 (1982) 400 ...
... I. V. Kozhevnikov, Russ. Chem. Rev. 56 (1987) 811 M. Misono, N. Mizuno, K. Katamura, A. Kasai, Y. Konishi, K. Sakata, T. Okuhara, Y. Yoneda, Bull. Chem. Soc. Jpn. 55 (1982) 400 ...
C - Science at St. Dominics
... Oxidation of a secondary alcohol Acidified sodium dichromate The ...
... Oxidation of a secondary alcohol Acidified sodium dichromate The ...
Alcohols/Wade: Reactions
... 10. Compound A is an optically active alcohol. Treatment with chromic acid converts A to a ketone B. In a separate reaction A is treated with PBr3, converting A to C. Compound D is purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. ...
... 10. Compound A is an optically active alcohol. Treatment with chromic acid converts A to a ketone B. In a separate reaction A is treated with PBr3, converting A to C. Compound D is purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. ...
Chemistry 220
... 3. Using the chemical shift table given on the next page, interpret the following NMR spectrum. Put your answer on the blank page which follows the table. In your answer, clearly explain how your proposed structure accounts for the observed number of signals, coupling, chemical shift and integratio ...
... 3. Using the chemical shift table given on the next page, interpret the following NMR spectrum. Put your answer on the blank page which follows the table. In your answer, clearly explain how your proposed structure accounts for the observed number of signals, coupling, chemical shift and integratio ...
honors final key
... a. How many moles of oxygen are consumed when 96.7 moles of hydrogen sulfide gas are burned, producing sulfur dioxide and water vapor in the process? =14.4 moles b. If 3.70 x 1023 molecules of oxygen react with excess benzene (C6H6), how many grams of water can be produced? =27g H2O c. 25.0 g Calciu ...
... a. How many moles of oxygen are consumed when 96.7 moles of hydrogen sulfide gas are burned, producing sulfur dioxide and water vapor in the process? =14.4 moles b. If 3.70 x 1023 molecules of oxygen react with excess benzene (C6H6), how many grams of water can be produced? =27g H2O c. 25.0 g Calciu ...
PowerPoint 프레젠테이션
... The Robinson’s synthesis of tropinone was hailed as revolutionary. This was to look at the target molecule and try to imagine how the molecule could be constructed from simpler chemical units. ...
... The Robinson’s synthesis of tropinone was hailed as revolutionary. This was to look at the target molecule and try to imagine how the molecule could be constructed from simpler chemical units. ...
Synthesis of Methyl Benzoate by Fisher Esterification
... equilibrium. To upset the equilibrium we can, by Le Chatelier's principle, increase the concentration of either the alcohol or acid, as noted above. If either one is doubled, the theoretical yield increases to 85%. When one is tripled, it goes to 90%. But note that in the example cited the boiling p ...
... equilibrium. To upset the equilibrium we can, by Le Chatelier's principle, increase the concentration of either the alcohol or acid, as noted above. If either one is doubled, the theoretical yield increases to 85%. When one is tripled, it goes to 90%. But note that in the example cited the boiling p ...
Lab Report: Qualitative Organic Analysis
... 6. Using the pKa and pKb values in you textbook and your knowledge of general chemistry. Write a chemical equation and calculate the equilibrium constant for the potential acid/base reaction at room temperature between benzoic acid and aniline. ...
... 6. Using the pKa and pKb values in you textbook and your knowledge of general chemistry. Write a chemical equation and calculate the equilibrium constant for the potential acid/base reaction at room temperature between benzoic acid and aniline. ...
Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
Organic Chemistry 1 1st Hour Exam Student ID # Name
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
The Baylis–Hillman reaction is an organic reaction of an aldehyde
... for easy epimerization of the nitro-substituted carbon atom, the Henry Reaction will typically produce a mixture of enantiomers or diastereomers. It is for this reason that explanations for stereoselectivity remain scarce without some modification. In recent years, research focus has shifted toward ...
... for easy epimerization of the nitro-substituted carbon atom, the Henry Reaction will typically produce a mixture of enantiomers or diastereomers. It is for this reason that explanations for stereoselectivity remain scarce without some modification. In recent years, research focus has shifted toward ...
PowerPoint
... methane, with water as a byproduct. The water that is produced can then react with CO in the water-gas shift reaction, equation (2). In addition, both CO and methane can decompose to form carbon as in equations (3) and (4). ...
... methane, with water as a byproduct. The water that is produced can then react with CO in the water-gas shift reaction, equation (2). In addition, both CO and methane can decompose to form carbon as in equations (3) and (4). ...
Oxidation-Reduction (Redox) Reactions
... H is typically +1 when it’s with nonmetals and –1 when it’s with metals. Oxygen and metals take precedence. H2O O has to be –2, so each H is +1. NaH Na has to be +1 (alkali metal), so H is –1. ...
... H is typically +1 when it’s with nonmetals and –1 when it’s with metals. Oxygen and metals take precedence. H2O O has to be –2, so each H is +1. NaH Na has to be +1 (alkali metal), so H is –1. ...
Nugget
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
HONORS: UNIT 2B: Antacids Below are the class objectives
... is a chemical reaction has occurred Practice converting from word equations to formula equations Define and predict products for synthesis (standard: binary ionic compound model) &decomposition reactions (binary ionic and metal carbonate) Define and predict products for single replacement reactions ...
... is a chemical reaction has occurred Practice converting from word equations to formula equations Define and predict products for synthesis (standard: binary ionic compound model) &decomposition reactions (binary ionic and metal carbonate) Define and predict products for single replacement reactions ...
Summary of Organic Compounds -Functional Groups and Reactions
... Hydrogen (hydrogenation) to form alcohols Controlled oxidation of aldehydes to form carboxylic acids Carboxylic acid + alcohol = ester + H2O ...
... Hydrogen (hydrogenation) to form alcohols Controlled oxidation of aldehydes to form carboxylic acids Carboxylic acid + alcohol = ester + H2O ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.