Carbohydrate Structure
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... Progress of single reaction can be represented by a single molar extent of reaction. What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
... Progress of single reaction can be represented by a single molar extent of reaction. What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
Chapter 11 Introduction to Organic Chemistry Part 2
... 2 (a) Although both structures A and C are the staggered conformations of 2-methylbutane and more stable than structures B and D, which are eclipsed conformations, structure A will be the most stable conformation because all the methyl groups and hydrogen atoms are farther apart from each other than ...
... 2 (a) Although both structures A and C are the staggered conformations of 2-methylbutane and more stable than structures B and D, which are eclipsed conformations, structure A will be the most stable conformation because all the methyl groups and hydrogen atoms are farther apart from each other than ...
SAMPLE PROBLEM
... FORWARD SYNTHESIS of retrosynthetic analysis 1. The synthesis begins with compound 12 available from a three-step synthesis starting with the (l) isomer of tartaric acid.2 Upon triflation the primary alcohols are activated to SN2 displacement by carbanions.4 In the sited example the sodium salt of a ...
... FORWARD SYNTHESIS of retrosynthetic analysis 1. The synthesis begins with compound 12 available from a three-step synthesis starting with the (l) isomer of tartaric acid.2 Upon triflation the primary alcohols are activated to SN2 displacement by carbanions.4 In the sited example the sodium salt of a ...
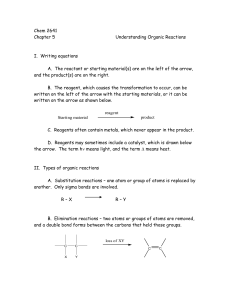
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
06 MC /08 MC /08 NMR
... only trans-l-4-dimethylcyclohexane. onlycrs-1-4-dimethylcyclohexane. both trans and cis-l -4-dimethylcyclohexane. It's impossible to tell. --'i ...
... only trans-l-4-dimethylcyclohexane. onlycrs-1-4-dimethylcyclohexane. both trans and cis-l -4-dimethylcyclohexane. It's impossible to tell. --'i ...
GR.12 ALCOHOL WITH KEY 06-07
... F. 1. The dehydration of the alcohol (A) in the presence of concentrated sulfuric acid leads to the formation of two alkene isomers. What is the compound (A)? Write the condensed structural formulas of the 2 alkene isomers. 2. The alcohol (A) is a liquid of boiling point t = 100oC, while the ether c ...
... F. 1. The dehydration of the alcohol (A) in the presence of concentrated sulfuric acid leads to the formation of two alkene isomers. What is the compound (A)? Write the condensed structural formulas of the 2 alkene isomers. 2. The alcohol (A) is a liquid of boiling point t = 100oC, while the ether c ...
Experiment 7-Reduction
... the two isomers are different and become diastereomeric. Thus, the amounts of each will not by equal. Chemists have been developing several chiral reducing agents, in combination with ligands, solvents and agents, to find the most efficient. One of the most common is L-tartaric acid, which selective ...
... the two isomers are different and become diastereomeric. Thus, the amounts of each will not by equal. Chemists have been developing several chiral reducing agents, in combination with ligands, solvents and agents, to find the most efficient. One of the most common is L-tartaric acid, which selective ...
Chapter 20 Amines-part 2
... Alkylation or acylation occurs mostly at carbon rather than nitrogen ...
... Alkylation or acylation occurs mostly at carbon rather than nitrogen ...
IOSR Journal of Applied Chemistry (IOSR-JAC) ISSN: 2278-5736.
... embrace aspects of both. It is implicated when a reaction selectivity at one functional group is needed in the presence of other functional group. When the principles of chemo selectivity are not applicable, the accompanying functional group must be protected [1]. Hydroxyl group of primary secondary ...
... embrace aspects of both. It is implicated when a reaction selectivity at one functional group is needed in the presence of other functional group. When the principles of chemo selectivity are not applicable, the accompanying functional group must be protected [1]. Hydroxyl group of primary secondary ...
Chap Thirteen: Alcohols
... ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configur ...
... ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configur ...
Outline_CH13_Klein
... ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configur ...
... ii. rxn w Thionyl chloride SOCl2 to form alkyl chlorides o SN2 and SN1 Mechanisms compete/ no rearrangement/ inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configur ...
Chapter 11 Review sheet Name
... reaction. If the change is caused by heat supplied to the reaction, the Greek symbol (9) is often written above the "yields" symbol in the equation. A chemical change in which a free element replaces and releases another element in a compound is called a(n) (10) reaction. A chemical change in which ...
... reaction. If the change is caused by heat supplied to the reaction, the Greek symbol (9) is often written above the "yields" symbol in the equation. A chemical change in which a free element replaces and releases another element in a compound is called a(n) (10) reaction. A chemical change in which ...
CHE 312 Exam III Review Sheet - Saint Leo University Faculty
... 4. Know the odd reactions for aniline, phenol, anisole, etc. and those of the sidechains including the main reaction reactions. 5. Know the reaction and mechanism for phenol with carbon dioxide in the presence of hydroxide ion. 6. Be able to explain how an ortho-, para- director works and how to ide ...
... 4. Know the odd reactions for aniline, phenol, anisole, etc. and those of the sidechains including the main reaction reactions. 5. Know the reaction and mechanism for phenol with carbon dioxide in the presence of hydroxide ion. 6. Be able to explain how an ortho-, para- director works and how to ide ...
Carbohydrate Structure
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
Carbohydrate Structure
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
... Simple Sugars • Cannot be broken down by mild acid hydrolysis • C3-9 (esp. 5 and 6) • Polyalcohols with aldehyde or ketone functional group • Many chiral compounds • C has tetrahedral bond angles ...
Grade 11 Chemistry Exam Review
... a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4(aq) + 3Ba(NO3)2(s) → Ba3(PO4)2(s) + 6NH4NO3(aq). d) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(aq). ...
... a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4(aq) + 3Ba(NO3)2(s) → Ba3(PO4)2(s) + 6NH4NO3(aq). d) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(aq). ...
Chemical Reactions
... Rules for Balancing • The only place you can change any number is the coefficient. • A coefficient is a number written in front of a chemical formula. • Don’t forget diatomic molecules. • Use the smallest ratio of coefficients possible. ...
... Rules for Balancing • The only place you can change any number is the coefficient. • A coefficient is a number written in front of a chemical formula. • Don’t forget diatomic molecules. • Use the smallest ratio of coefficients possible. ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.