Cl 2
... In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. • An example of excess and limiting reagents is making muffins. You have more than enough flour, butter, water, and eggs. However, you only have one cup of sugar. The number of muffi ...
... In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. • An example of excess and limiting reagents is making muffins. You have more than enough flour, butter, water, and eggs. However, you only have one cup of sugar. The number of muffi ...
5. Coenzyme HAD+ is derived
... important molecules. Classification of organic reactions by type and mechanism. Reactivity of hydrocarbons. Radical substitution reaction. Of electrophilic substitution and addition. Acidity and basicity of organic compounds. Nucleophilic substitution at saturated carbon atoms. Reactivity of carbony ...
... important molecules. Classification of organic reactions by type and mechanism. Reactivity of hydrocarbons. Radical substitution reaction. Of electrophilic substitution and addition. Acidity and basicity of organic compounds. Nucleophilic substitution at saturated carbon atoms. Reactivity of carbony ...
Preparation of ethers
... tions of temperature and pressure. The reactants compris The above example was repeated by charging 64 g. ing an ole?n and an alcohol are continuously charged 30 (2.0 mole) of methyl alcohol and 5 g. of a catalyst com~ to this reactor through separate lines, or if so desired, prising vanadium pentox ...
... tions of temperature and pressure. The reactants compris The above example was repeated by charging 64 g. ing an ole?n and an alcohol are continuously charged 30 (2.0 mole) of methyl alcohol and 5 g. of a catalyst com~ to this reactor through separate lines, or if so desired, prising vanadium pentox ...
lecture 7 reductive eliminations
... product was determined by carbonylation to give the metal acyl followed by methanolysis to give the ester. • Both of these reactions are known to leave the configuration at carbon unchanged, and the configuration of the ester can be determined unambiguously from the measured optical rotation of the ...
... product was determined by carbonylation to give the metal acyl followed by methanolysis to give the ester. • Both of these reactions are known to leave the configuration at carbon unchanged, and the configuration of the ester can be determined unambiguously from the measured optical rotation of the ...
Unit 13: Organic Chemistry
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
naming of ethers
... Ethers are characterized by the functional group R – O – R, two alkyl groups joined by an oxygen atom. Due to its shape ethers have a slight polarity and due to the absence of hydrogen bonding, ethers have much lower boiling points than alcohols. Consider the hydrogen bonding of the following compou ...
... Ethers are characterized by the functional group R – O – R, two alkyl groups joined by an oxygen atom. Due to its shape ethers have a slight polarity and due to the absence of hydrogen bonding, ethers have much lower boiling points than alcohols. Consider the hydrogen bonding of the following compou ...
Section 2 Hydrocarbons Chapter 22
... • An addition reaction is one in which two parts of a molecule are added to an unsaturated molecule, increasing the saturation of the molecule. • example: hydrogenation, the addition of hydrogen atoms to an unsaturated molecule. • Vegetable oils contain long chains of carbon atoms with many double b ...
... • An addition reaction is one in which two parts of a molecule are added to an unsaturated molecule, increasing the saturation of the molecule. • example: hydrogenation, the addition of hydrogen atoms to an unsaturated molecule. • Vegetable oils contain long chains of carbon atoms with many double b ...
Stoichiometry Notes
... 1. Equimolar solutions of sodium chloride and silver nitrate are mixed. 2. A solution of lead nitrate is added to a solution of sodium iodide. ...
... 1. Equimolar solutions of sodium chloride and silver nitrate are mixed. 2. A solution of lead nitrate is added to a solution of sodium iodide. ...
Carbohidratos
... ReacKons of Monosaccharides • Monosaccharides are generally soluble in water. WHY? • To improve their solubility in organic solvents, the hydroxyl groups can be acetylated. ...
... ReacKons of Monosaccharides • Monosaccharides are generally soluble in water. WHY? • To improve their solubility in organic solvents, the hydroxyl groups can be acetylated. ...
CHAPTER 14 CHEMICAL KINETICS
... is given by Equations (14.3) and (14.4) of the text. We are asked to determine the time required for 95% of the phosphine to decompose. If we initially have 100% of the compound and 95% has reacted, then what is left must be (100% 95%), or 5%. Thus, the ratio of the percentages will be equal to th ...
... is given by Equations (14.3) and (14.4) of the text. We are asked to determine the time required for 95% of the phosphine to decompose. If we initially have 100% of the compound and 95% has reacted, then what is left must be (100% 95%), or 5%. Thus, the ratio of the percentages will be equal to th ...
chapter 21
... Strategy: We are given information as to how the concentrations of X2, Y, and Z affect the rate of the reaction and are asked to determine the rate law. We assume that the rate law takes the form rate k[X2]x[Y]y[Z]z How do we use the information to determine x, y, and z? Solution: Since the reacti ...
... Strategy: We are given information as to how the concentrations of X2, Y, and Z affect the rate of the reaction and are asked to determine the rate law. We assume that the rate law takes the form rate k[X2]x[Y]y[Z]z How do we use the information to determine x, y, and z? Solution: Since the reacti ...
Chapter 17 Amines
... 756; M. N. Khan, J. Org. Chem. 61, 8063 (1996). Stereoselectivity: A. Kubo et al., Tetrahedron Letters 37, ...
... 756; M. N. Khan, J. Org. Chem. 61, 8063 (1996). Stereoselectivity: A. Kubo et al., Tetrahedron Letters 37, ...
Introduction to Organic Chemistry 2 ed William H. Brown
... • Oxygen is more electronegative than carbon (3.5 vs 2.5) and, therefore, a C=O group is polar + C O • aldehydes and ketones have higher boiling points and are more soluble in water than nonpolar compounds of ...
... • Oxygen is more electronegative than carbon (3.5 vs 2.5) and, therefore, a C=O group is polar + C O • aldehydes and ketones have higher boiling points and are more soluble in water than nonpolar compounds of ...
Chapter-16A
... Fischer esterification is an equilibrium reaction • by careful control of experimental conditions, it is possible to prepare esters in high yield • if the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the right • a key intermediate in Fi ...
... Fischer esterification is an equilibrium reaction • by careful control of experimental conditions, it is possible to prepare esters in high yield • if the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the right • a key intermediate in Fi ...
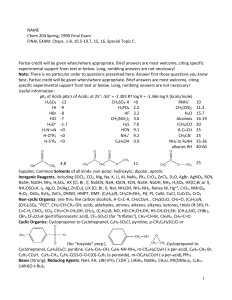
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1
... 3.(09) Compound (VII) was required for a diterpene synthesis (JACS, 103 222(1981)). (VII) can be synthesized from (VI) but only if a "protection-deprotection" procedure, involving a temporary "mask", is incorporated into the sequence of reactions from (VI) to (VII). Outline the reactions required, i ...
... 3.(09) Compound (VII) was required for a diterpene synthesis (JACS, 103 222(1981)). (VII) can be synthesized from (VI) but only if a "protection-deprotection" procedure, involving a temporary "mask", is incorporated into the sequence of reactions from (VI) to (VII). Outline the reactions required, i ...
4 ORGANIC CHEMISTRY: STRUCTURE AND NOMENCLATURE
... O1.4 ROTATION AROUND COC BONDS It is easy to fall into the trap of thinking about the ethane molecule as if it were static. Nothing could be further from the truth. At room temperature, the average velocity of an ethane molecule is about 500 m/s—more than twice the speed of a Boeing 747. While it mo ...
... O1.4 ROTATION AROUND COC BONDS It is easy to fall into the trap of thinking about the ethane molecule as if it were static. Nothing could be further from the truth. At room temperature, the average velocity of an ethane molecule is about 500 m/s—more than twice the speed of a Boeing 747. While it mo ...
word - My eCoach
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
CHEM 203 Material
... state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that is, it will tend to react with electron acceptors. Likewise, one may predict that hypothetical reactions ...
... state of –4. This produces a significant concentration of electronic density around the C atom. One may predict that the C atom in methane will behave as an electron donor in its reactions; that is, it will tend to react with electron acceptors. Likewise, one may predict that hypothetical reactions ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.