* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Carbohidratos
Survey
Document related concepts
Transcript
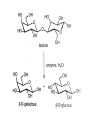
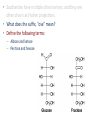
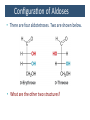
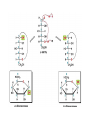
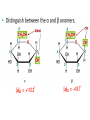
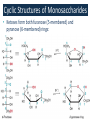
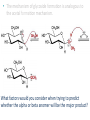
Carbohidratos IntroducKon to Carbohydrates • Carbohydrates (sugars) are abundant in nature: – They are high energy biomolecules. – They provide structural rigidity for organisms (plants, crustaceans, etc.). – The polymer backbone on which DNA and RNA are assembled contains sugars. • The term, carbohydrate, evolved to describe the formula for such molecules: Cx(H2O)x. • Carbohydrates are NOT true hydrates. WHY? • Carbohydrates (sugars) are polyhydroxy aldehydes or ketones. – Consider glucose, which is made by plants: – Is glucose a polyhydroxy aldehyde or ketone? ClassificaKon of Monosaccharides • Saccharides have multiple chiral centers, and they are often drawn as Fischer projecKons. – Designate each chirality center in glucose as either R or S. • Saccharides have mulKple chiral centers, and they are often drawn as Fischer projecKons. • What does the suffix, “ose” mean? • Define the following terms: – Aldose and ketose – Pentose and hexose • Glyceraldehyde is a monosaccharide with one chirality center. – Natural glyceraldehyde is dextrorotatory (D): it rotates plane polarized light in the clockwise direcKon. ClassificaKon of Monosaccharides • Naturally occurring larger sugars can be broken down into glyceraldehyde by degradaKon. • Such sugars are often called D-‐sugars. ClassificaKon of Monosaccharides • Recall that dextrorotatory versus levorotatory rotation cannot be predicted by the R or S configuraKon. • Here, D no longer refers to dextrorotatory. Rather it refers to the R configuraKon at the chiral carbon farthest from the carbonyl. ConfiguraKon of Aldoses • There are four aldotetroses. Two are shown below. • What are the other two structures? ConfiguraKon of Aldoses • Aldopentoses have three chirality centers. The number of isomers will be 23. • Recall the 2n rule. • The D-‐sugars are naturally occurring. ConfiguraKon of Aldoses • Ribose is a key building block of RNA. – WHAT is RNA? • Arabinose is found in plants. • Xylose is found in wood. ConfiguraKon of Aldoses • • • • Based on the 2n rule, how many aldohexoses are there? How many of the aldohexoses are D isomers. Glucose is the most common aldohexose. Mannose and galactose are also common. ConfiguraKon of Ketoses • Relevant ketoses have between three and six carbons. • For each naturally occurring D isomer, there is an L enanKomer. ConfiguraKon of Ketoses Cyclic Structures of Monosaccharides • Carbonyls can be a[acked by alcohols to form hemiacetals. – The intramolecular reacKon is generally favored for 5 and 6-‐ membered rings. WHY? Cyclic Structures of Monosaccharides • For the following compound, draw the mechanism and resulKng product that results from acid catalyzed ring-‐ closing hemiacetal formaKon. Cyclic Structures of Monosaccharides • Monosaccharides, like glucose, can also undergo ring-‐closing hemiacetal formaKon. • The equilibrium greatly favors the closed form called pyranose. Anomeric effect • Which would you predict to be more stable? – Beta 67% , alpha 33%, open 0.01% 9 Cyclic Structures of Monosaccharides • Ketoses form both furanose (5-‐membered) and pyranose (6-‐membered) rings: Cyclic Structures of Monosaccharides 70% β 2% α 0.7% 23%-β 5%-α • The equilibrium concentrations in water are above. Cyclic Structures of Monosaccharides • The furanose form takes part in most biochemical reacKons. ReacKons of Monosaccharides • Monosaccharides are generally soluble in water. WHY? • To improve their solubility in organic solvents, the hydroxyl groups can be acetylated. • WHY is pyridine added to the reacKon? • How might acetylaKon help in purificaKon efforts. ReacKons of Monosaccharides • Monosaccharides can also be converted to ethers via the Williamson ether synthesis. • Ether linkages are more robust than ester linkages. WHY? ReacKons of Monosaccharides • When treated with an excess of an alcohol, the hemiacetal equilibrium can be shifted to give an acetal. • When a sugar is used, alpha and beta glycosides are formed. ReacKons of Monosaccharides • The mechanism of glycoside formaKon is analogous to the acetal formaKon mechanism. • Only the anomeric hydroxyl group is replaced. • The mechanism of glycoside formation is analogous to the acetal formaKon mechanism. What factors would you consider when trying to predict whether the alpha or beta anomer will be the major product? ReacKons of Monosaccharides • Under strongly basic conditions, glucose and mannose interconvert. • Mannose and glucose are epimers because they only differ in the configuraKon of one carbon center. ReacKons of Monosaccharides • Monosaccharides can be reduced to ALDITOLs shifing the equilibrium to the right. HOW? – D-‐sorbitol or D-‐glucitol are sugar subsKtutes. Reducing sugars • If the sugar has an –OH a[ached to the anomeric carbon, then the sugar is a reducing sugar • If it has –OR, then it is not a reducing sugar ReacKons of Monosaccharides Disaccharides • Disaccharides form when two sugars connect through a glycosidic linkage. – The 1 à 4 glycosidic linkage is most common. – The bottom ring is capable of mutarotaKon at its anomeric posiKon. – Because the anomeric posiKon of the bottom ring is a HEMIACETAL rather than an acetal, it is in equilibrium with the open form. Thus, maltose is a reducing sugar. Disaccharides • Cellobiose is similar to maltose. WHAT are the differences? • Will cellobiose be a reducing sugar? Disaccharides • Lactose is another disaccharide. • Some people have trouble digesKng lactose.