Iodine and Lipase Based Green Oxidation Technology
... recycled are the desired ones. 1.1.4 Recovery and recycling Ease of recovery of reagents and catalysts is important for two reasons: (1) complete removal of catalyst is necessary to prevent contamination of the product. (2) It allows the recycling of the catalyst. Heterogenization is a commo ...
... recycled are the desired ones. 1.1.4 Recovery and recycling Ease of recovery of reagents and catalysts is important for two reasons: (1) complete removal of catalyst is necessary to prevent contamination of the product. (2) It allows the recycling of the catalyst. Heterogenization is a commo ...
bonding
... 5.14: Dehydrohalogenation of Alkyl Halides - loss of H and X from adjacent carbons of an alkyl halide to form an alkene. Elimination of alkyl halides is affected by base, often the conjugate bases of alcohols (alkoxides), by an E2 mechanism The reaction follows Zaitsev's rule in that the most stabl ...
... 5.14: Dehydrohalogenation of Alkyl Halides - loss of H and X from adjacent carbons of an alkyl halide to form an alkene. Elimination of alkyl halides is affected by base, often the conjugate bases of alcohols (alkoxides), by an E2 mechanism The reaction follows Zaitsev's rule in that the most stabl ...
Exam Review Packet Table of Contents
... (Δx) (Δp) [greater than or equal to] h-‐bar (or h / 2π) h = Planck's constant Δx = uncertainty in position Δp = uncertainty in momentum Notes: 1 point is given for the notion of simulta ...
... (Δx) (Δp) [greater than or equal to] h-‐bar (or h / 2π) h = Planck's constant Δx = uncertainty in position Δp = uncertainty in momentum Notes: 1 point is given for the notion of simulta ...
8 theoretical problems 2 practical problems
... give the same osazone and therefore have identical stereochemistry at C-3, C-4, and C-5 (and C-6). A and B are also different from compound 1 (i.e. D-mannose) yet give the same osazone, and thus one of them must be the C-2 epimer of D-mannose (i.e. D-glucose) and the other must be the corresponding ...
... give the same osazone and therefore have identical stereochemistry at C-3, C-4, and C-5 (and C-6). A and B are also different from compound 1 (i.e. D-mannose) yet give the same osazone, and thus one of them must be the C-2 epimer of D-mannose (i.e. D-glucose) and the other must be the corresponding ...
Chap15 - Bakersfield College
... Predicting the Direction of Reaction • How could we predict the direction in which a reaction at non-equilibrium conditions will shift to reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The react ...
... Predicting the Direction of Reaction • How could we predict the direction in which a reaction at non-equilibrium conditions will shift to reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The react ...
Formatting Blackline Masters
... Note: A represents the central atom in the molecule. B represents atoms bonded to the central atom. B can be identical atoms or different atoms. Directions: 1. Find the other students who have the same color balloons as you. Have someone inflate a balloon as much as possible without popping it. Infl ...
... Note: A represents the central atom in the molecule. B represents atoms bonded to the central atom. B can be identical atoms or different atoms. Directions: 1. Find the other students who have the same color balloons as you. Have someone inflate a balloon as much as possible without popping it. Infl ...
High-Oxidation-State Palladium Catalysis: New Reactivity for
... diacetate, PhI(OAc)2 , the corresponding acetoxylation product is formed in acetonitrile, whereas selective alkoxylation reactions are possible in alcoholic solvents. The C H functionalization of single arenes proceeds with high selectivity[22] for the ortho position; thus, this reaction provides un ...
... diacetate, PhI(OAc)2 , the corresponding acetoxylation product is formed in acetonitrile, whereas selective alkoxylation reactions are possible in alcoholic solvents. The C H functionalization of single arenes proceeds with high selectivity[22] for the ortho position; thus, this reaction provides un ...
Chapter 1: Chemistry: The Study of Change
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
Polymerization Lab
... 5) Unlike addition polymerization condensation polymers can propagate from both ends of the molecule. Gather together the monomers for Polymer A and begin by cutting along the dotted lines on one of the monomers. 6) Cut along the dotted lines on a second monomer and fit these two together. a. What f ...
... 5) Unlike addition polymerization condensation polymers can propagate from both ends of the molecule. Gather together the monomers for Polymer A and begin by cutting along the dotted lines on one of the monomers. 6) Cut along the dotted lines on a second monomer and fit these two together. a. What f ...
Polymerization Lab
... Polymers (Greek-POLY...many and MEROS...parts) have existed since the beginning of life. Both "natural" and "synthetic" polymers are an integral part of our life. Most of the natural and synthetic materials with which we come in contact are wholly or partly polymeric in nature (Carboyhydrates, cellu ...
... Polymers (Greek-POLY...many and MEROS...parts) have existed since the beginning of life. Both "natural" and "synthetic" polymers are an integral part of our life. Most of the natural and synthetic materials with which we come in contact are wholly or partly polymeric in nature (Carboyhydrates, cellu ...
Homework1-4-Answers
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
Full-Text PDF
... Oxidative desulfurization-fluorination of xanthates is now the most attractive method for primary alkyl trifluoromethyl ether synthesis. At least 42 publications concerning this reaction are cited in Reaxys. N-Bromo- [13] or N-iodosuccinimide [14], dibromodimethylhidantoine [15,16], and IF5 [17] are ...
... Oxidative desulfurization-fluorination of xanthates is now the most attractive method for primary alkyl trifluoromethyl ether synthesis. At least 42 publications concerning this reaction are cited in Reaxys. N-Bromo- [13] or N-iodosuccinimide [14], dibromodimethylhidantoine [15,16], and IF5 [17] are ...
Problem 1-2 - IPN-Kiel
... To determine the iron(III) content in a solution it is precipitated with ammonia, filtered through ashfree filters, washed with water and at the end with ammonium nitrate solution. The filter with the precipitate is given into a porcelain crucible and heated with a Bunsen burner, at first slowly and ...
... To determine the iron(III) content in a solution it is precipitated with ammonia, filtered through ashfree filters, washed with water and at the end with ammonium nitrate solution. The filter with the precipitate is given into a porcelain crucible and heated with a Bunsen burner, at first slowly and ...
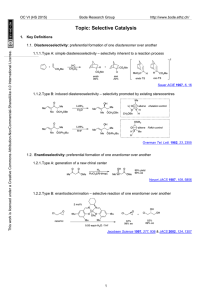
Changing counterion can switch the preference for selective 1,2
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
... Another example of a selectivity-controlled reaction of alkenes is hydroformylation via the use of scaffolding ligands. These bind covalently and reversibly to the substrate, leading to a temporarily intramolecular transformation that can lead to dramatically improved and reversed selectivity with s ...
Ch-9-Carboxylic Acids and their derivatives new
... • Find the longest continuous carbon chain contains the COOH group to get the name of the parent hydrocarbon, the ending -e is replaced by the suffix –oic acid. • Number the chain starting with the carbon of COOH group as C-1 • If there are substituents identify their names and positions and list th ...
... • Find the longest continuous carbon chain contains the COOH group to get the name of the parent hydrocarbon, the ending -e is replaced by the suffix –oic acid. • Number the chain starting with the carbon of COOH group as C-1 • If there are substituents identify their names and positions and list th ...
U3 Student Workbook - The Connected Chemistry Curriculum
... at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical formulas for the reactants and products. In the final activity of the lesson, students can independently practice balancing equations. SWBAT (Student will be able to) • Know t ...
... at ten different reactions. In each of the reactions, students will create submicroscopic sketches and balance the chemical formulas for the reactants and products. In the final activity of the lesson, students can independently practice balancing equations. SWBAT (Student will be able to) • Know t ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
M.Sc. Chemistry - Periyar University
... To learn about the mechanism of aliphatic and aromatic nucleophilic substitution reactions and aromatic electrophilic substitution reactions. To learn about the structural elucidation of alkaloids flavones and isoflavones. ...
... To learn about the mechanism of aliphatic and aromatic nucleophilic substitution reactions and aromatic electrophilic substitution reactions. To learn about the structural elucidation of alkaloids flavones and isoflavones. ...
7.1 CHEMICAL SYSTEMS IN EQUILIBRIUM: Dynamic Equilibrium in
... The proportions of nitrogen and hydrogen The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means t ...
... The proportions of nitrogen and hydrogen The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means t ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
Transition Metal Catalyzed Selective Oxidation of Sugars and Polyols
... The oxidation of alicyclic vicinal diols to α-hydroxy ketones was investigated as a model system for the selective oxidation of a secondary hydroxyl group in sugar substrates to keto-sugars. The challenge was to develop a method that allows selective oxidation, yet prevents over-oxidation to the dio ...
... The oxidation of alicyclic vicinal diols to α-hydroxy ketones was investigated as a model system for the selective oxidation of a secondary hydroxyl group in sugar substrates to keto-sugars. The challenge was to develop a method that allows selective oxidation, yet prevents over-oxidation to the dio ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.