Transition Metals
... oxidised to Cl2 by MnO4It cannot be nitric acid as this is an oxidising agent. It oxidises Fe2+ to Fe3+ It cannot be conc H2SO4 as this is an oxidising agent. It cannot be ethanoic acid as this is a weak acid and not supply the large amount of acid needed (8H+) ...
... oxidised to Cl2 by MnO4It cannot be nitric acid as this is an oxidising agent. It oxidises Fe2+ to Fe3+ It cannot be conc H2SO4 as this is an oxidising agent. It cannot be ethanoic acid as this is a weak acid and not supply the large amount of acid needed (8H+) ...
Title Decomposition studies of isopropanol in a
... used in the experiments. Standard calibration gases (Airgas and Air Liquide) are flowed through the sampling system to calibrate the GC-FID analyses for all other species (permanent gases, and hydrocarbons with carbon number <6). The analyses of a standard gas mixture of known composition (Airgas a ...
... used in the experiments. Standard calibration gases (Airgas and Air Liquide) are flowed through the sampling system to calibrate the GC-FID analyses for all other species (permanent gases, and hydrocarbons with carbon number <6). The analyses of a standard gas mixture of known composition (Airgas a ...
Practice Problems in Biomedical Organic Chemistry
... in terms of relating this knowledge to practical, everyday problems. It is unfortunate that organic chemistry has been distorted in many peoples’ minds to become a roadblock obstructing their way to a degree. Instead of ‘doing time’ in organic, we hope that students will embrace this opportunity to ...
... in terms of relating this knowledge to practical, everyday problems. It is unfortunate that organic chemistry has been distorted in many peoples’ minds to become a roadblock obstructing their way to a degree. Instead of ‘doing time’ in organic, we hope that students will embrace this opportunity to ...
kcse chemistry questions
... Write an equation for the reaction between potassium and compound C4H10O. During the production of hydrogen iodide, hydrogen reacts with iodine according to the equation: H2 (g) + I2 (g) 2HI (g); 52.0 kJ Explain how the following would affect the yield of hydrogen iodide: a) Increase in temperature ...
... Write an equation for the reaction between potassium and compound C4H10O. During the production of hydrogen iodide, hydrogen reacts with iodine according to the equation: H2 (g) + I2 (g) 2HI (g); 52.0 kJ Explain how the following would affect the yield of hydrogen iodide: a) Increase in temperature ...
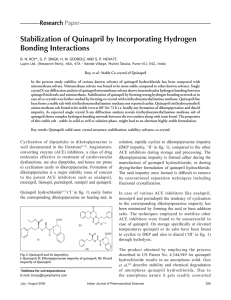
Stabilization of Quinapril by Incorporating Hydrogen Bonding
... salt (quinapril benzyl ester maleate salt) 25 g (0.0388 mol) was dissolved in a mixture of 125 ml water and 125 ml dichloromethane. The pH of the solution was adjusted between 7.5 and 8.5 by addition of aqueous ammonia. Reaction mixture was stirred for 30 min, the organic phase separated and washed ...
... salt (quinapril benzyl ester maleate salt) 25 g (0.0388 mol) was dissolved in a mixture of 125 ml water and 125 ml dichloromethane. The pH of the solution was adjusted between 7.5 and 8.5 by addition of aqueous ammonia. Reaction mixture was stirred for 30 min, the organic phase separated and washed ...
Chemical Reactions and Stoichiometry
... Combustion analysis (which we saw in the previous chapter) employs a chemical reaction, a process in which one or more substances are converted into one or more different ones. Compounds form and change through chemical reactions. Water can be made by the reaction of hydrogen with oxygen. A combusti ...
... Combustion analysis (which we saw in the previous chapter) employs a chemical reaction, a process in which one or more substances are converted into one or more different ones. Compounds form and change through chemical reactions. Water can be made by the reaction of hydrogen with oxygen. A combusti ...
Abstract Isomorphous substitution of Al or Si in zeolites/molecular
... peroxo-radical which abstracts a H atom from the hydrocarbon molecule to give a carbon radical, which is further hydroxylated to alcohol is proposed. Vanadium silicates are active in the hydroxylation of benzene. The product distribution contains phenol and para-benzoquinone (PBQ). Formation of PBQ ...
... peroxo-radical which abstracts a H atom from the hydrocarbon molecule to give a carbon radical, which is further hydroxylated to alcohol is proposed. Vanadium silicates are active in the hydroxylation of benzene. The product distribution contains phenol and para-benzoquinone (PBQ). Formation of PBQ ...
Ch. 24 - Organic Compounds
... engineers and some impressive special effects. However, the slime that dripped off of the actors was no illusion. The movie producers needed a way to manufacture real slime. They turned to the special effects artists, who relied on chemical engineers to manufacture genuine slime. The engineers were ...
... engineers and some impressive special effects. However, the slime that dripped off of the actors was no illusion. The movie producers needed a way to manufacture real slime. They turned to the special effects artists, who relied on chemical engineers to manufacture genuine slime. The engineers were ...
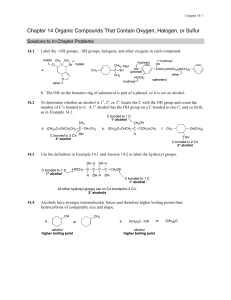
Chapter 14 Solutions
... a. CFCs are chlorofluorocarbons with a general formula of CFxCl4 – x; HCFCs have fluorine, chlorine, and hydrogen bonded to carbon; and HFCs have only hydrogen and fluorine bonded to carbon. b. CFCs destroy the ozone layer whereas HCFCs and HFCs decompose more readily before ascending to the ozone l ...
... a. CFCs are chlorofluorocarbons with a general formula of CFxCl4 – x; HCFCs have fluorine, chlorine, and hydrogen bonded to carbon; and HFCs have only hydrogen and fluorine bonded to carbon. b. CFCs destroy the ozone layer whereas HCFCs and HFCs decompose more readily before ascending to the ozone l ...
Scientific Jury of the 30th International
... and 3rd row elements in compounds with halogens and in oxoanions compounds of nonmetals with other oxidation states the preferred oxidation states are Sn(II), ...
... and 3rd row elements in compounds with halogens and in oxoanions compounds of nonmetals with other oxidation states the preferred oxidation states are Sn(II), ...
Pirogov National Medical Univercity of Vinnitsa
... 2. If skin solutions of alkali damaged area, washed with water, but in fact dilute acetic acid, citric acid, or a saturated solution of boric acid. 3. If skin phenol, bromine, and similar substances should immediately wash the damaged area relevant organic solvents (alcohol, ether, etc.). 4. In pois ...
... 2. If skin solutions of alkali damaged area, washed with water, but in fact dilute acetic acid, citric acid, or a saturated solution of boric acid. 3. If skin phenol, bromine, and similar substances should immediately wash the damaged area relevant organic solvents (alcohol, ether, etc.). 4. In pois ...
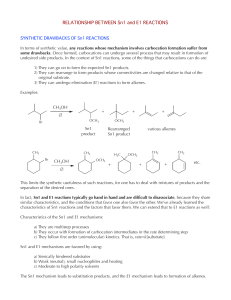
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... H Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat ...
... H Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat ...
Aldehydes and Ketones The Carbonyl Group
... Other Nomenclature Rules • In cyclic ketones, the carbonyl group is always numbered “1”; this does not need to be included in the name. The numbering continues clockwise or counterclockwise to give the lowest number for the next substituent. • Molecules with more than one ketone group are named by p ...
... Other Nomenclature Rules • In cyclic ketones, the carbonyl group is always numbered “1”; this does not need to be included in the name. The numbering continues clockwise or counterclockwise to give the lowest number for the next substituent. • Molecules with more than one ketone group are named by p ...
379 - FTP
... A disproportion reaction occurs rapidly in water forming hypochlorous acid: Cl2 (aq) → H+ + Cl¯ + HOCl The equilibrium constant, K, for this reaction at 25°C is 4.2x10–4. The standard redox potentials for the following two reactions: H+ + HOCl + e¯ → ½Cl2 (g) + H2O 2H+ + 2Cl¯ + ½O2 → Cl2 + H2O are 1 ...
... A disproportion reaction occurs rapidly in water forming hypochlorous acid: Cl2 (aq) → H+ + Cl¯ + HOCl The equilibrium constant, K, for this reaction at 25°C is 4.2x10–4. The standard redox potentials for the following two reactions: H+ + HOCl + e¯ → ½Cl2 (g) + H2O 2H+ + 2Cl¯ + ½O2 → Cl2 + H2O are 1 ...
Title Several Reactions of Isocyanide and Related Compounds
... (Ia) and (Ib). Carbon monoxide (II) and carbene (III) are characterized also by lonepair electrons on a carbon atom. ...
... (Ia) and (Ib). Carbon monoxide (II) and carbene (III) are characterized also by lonepair electrons on a carbon atom. ...
Graphene-Catalyzed Direct Friedel–Crafts Alkylation Reactions
... produce valuable diarylalkane products21 in high yields and excellent regioselectivity. This process constitutes the first general application of graphenes to promote direct C−C bond formation, utilizing polar functional groups anchored on the GO surface,23−30 which may open the door for industrial a ...
... produce valuable diarylalkane products21 in high yields and excellent regioselectivity. This process constitutes the first general application of graphenes to promote direct C−C bond formation, utilizing polar functional groups anchored on the GO surface,23−30 which may open the door for industrial a ...
The behaviour of esters in the presence of
... compounds, which were the dominant products generated by pyrolysis (cf. Fig. l), and the abundant presence of methoxy and hydroxy compounds clearly shows that pyrolysis has occurred not at all or only to a minor extent. Most of the products can be explained by assuming a hydrolysis reaction followed ...
... compounds, which were the dominant products generated by pyrolysis (cf. Fig. l), and the abundant presence of methoxy and hydroxy compounds clearly shows that pyrolysis has occurred not at all or only to a minor extent. Most of the products can be explained by assuming a hydrolysis reaction followed ...
Chemistry: Percent Yield
... 12: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC sy ...
... 12: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC sy ...
AP Chemistry Notes and Worksheets 2014
... o Shows that each element consists of a certain type of atom and that compounds were formed from specific combinations of atoms. 2.3 Dalton’s Atomic Theory Dalton's Atomic Theory -1808 o Each element is made up of particles called atoms. o Atoms in an element are all identical and are different th ...
... o Shows that each element consists of a certain type of atom and that compounds were formed from specific combinations of atoms. 2.3 Dalton’s Atomic Theory Dalton's Atomic Theory -1808 o Each element is made up of particles called atoms. o Atoms in an element are all identical and are different th ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.