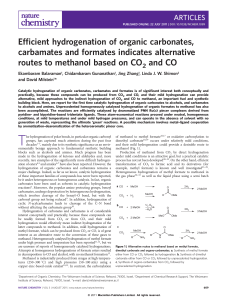
Dehydration of t-Amyl Alcohol (2-Methyl-2
... If a good nucleophile is present, such as: Cl-, Br-, I- (which would come from using strong acids HCl, HBr and HI respectively) it will form a new bond at the positive carbon. This is a nucleophilic substitution reaction. If the acid's conjugate base is a poor nucleophile such as HSO4- (which would ...
... If a good nucleophile is present, such as: Cl-, Br-, I- (which would come from using strong acids HCl, HBr and HI respectively) it will form a new bond at the positive carbon. This is a nucleophilic substitution reaction. If the acid's conjugate base is a poor nucleophile such as HSO4- (which would ...
Tests
... Chemistry is the study of the ________________ of matter and the __________ that matter undergoes. Matter is anything that has ___________ and takes up space. A _______________ would study the structure of the hemoglobin molecule and how it transports oxygen. An organic chemistry works mainly with _ ...
... Chemistry is the study of the ________________ of matter and the __________ that matter undergoes. Matter is anything that has ___________ and takes up space. A _______________ would study the structure of the hemoglobin molecule and how it transports oxygen. An organic chemistry works mainly with _ ...
Ch 16 Power Point
... • The enthalpy change that occurs during the complete combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H ...
... • The enthalpy change that occurs during the complete combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H ...
Ch14_PT MULTIPLE CHOICE. Choose the one alternative that best
... A) a carboxylic acid. B) an aldehyde. C) a ketone. D) an alkene. E) no reaction. 52) Gentle oxidation of a primary alcohol will produce A) an ether. B) an alkene. C) a ketone. D) an aldehyde. E) a carboxylic acid. ...
... A) a carboxylic acid. B) an aldehyde. C) a ketone. D) an alkene. E) no reaction. 52) Gentle oxidation of a primary alcohol will produce A) an ether. B) an alkene. C) a ketone. D) an aldehyde. E) a carboxylic acid. ...
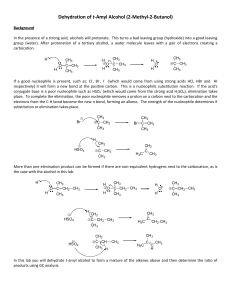
Efficient hydrogenation of organic carbonates, carbamates and
... alcohol, without cleavage of the benzyl–O bond. Thus, upon treatment of benzyl morpholine-4-carboxylate with H2 (10 atm) at 110 8C in dry THF for 52 h with a catalytic amount of 2 (1 mol%), 88% of benzyl alcohol, 87% of morpholine and 81% of methanol were obtained (Fig. 2a). It is important to note ...
... alcohol, without cleavage of the benzyl–O bond. Thus, upon treatment of benzyl morpholine-4-carboxylate with H2 (10 atm) at 110 8C in dry THF for 52 h with a catalytic amount of 2 (1 mol%), 88% of benzyl alcohol, 87% of morpholine and 81% of methanol were obtained (Fig. 2a). It is important to note ...
effect of inorganic ions on the oxidation of dichlorvos insecticide with
... This study analyzes the oxidation of dichlorvos with Fenton‘s reagent in solutions containing various ions. Results show that the larger the added amount of ferrous ions, the higher the elimination rate of dichlorvos and the oxidization rate after the addition of ferric ions is far smaller than that ...
... This study analyzes the oxidation of dichlorvos with Fenton‘s reagent in solutions containing various ions. Results show that the larger the added amount of ferrous ions, the higher the elimination rate of dichlorvos and the oxidization rate after the addition of ferric ions is far smaller than that ...
Fatty Acids - dan
... • Typical saturated fatty acids are tightly packed together • cis double bonds prevent good alignment of molecules in unsaturated fatty acids leading to poor packing • Double bonds lower melting point relative to saturated acid ...
... • Typical saturated fatty acids are tightly packed together • cis double bonds prevent good alignment of molecules in unsaturated fatty acids leading to poor packing • Double bonds lower melting point relative to saturated acid ...
AS/A level
... The activation energy of the reaction can be calculated by finding the value of the rate constant, k, at a series of different temperatures, T. The following graph shows a plot of ln k against 1/T. The gradient, m, of the line is related to the activation energy, Ea. ...
... The activation energy of the reaction can be calculated by finding the value of the rate constant, k, at a series of different temperatures, T. The following graph shows a plot of ln k against 1/T. The gradient, m, of the line is related to the activation energy, Ea. ...
tro2_ppt_lecture_04 - Louisiana Tech University
... Determine how many grams of glucose (C6H6O6) would be produced by the plant if 37.8 grams of CO2 is consumed. 1. Need a balanced equation: 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how many moles of C6H6 ...
... Determine how many grams of glucose (C6H6O6) would be produced by the plant if 37.8 grams of CO2 is consumed. 1. Need a balanced equation: 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how many moles of C6H6 ...
Chapter 15. α-AMINO ACIDS, PEPTIDES AND PROTEINS
... Amino acids can be classified as neutral, acidic, or basic, depending on the nature of their side chain, the R substituent. Most amino acids (fifteen of the twenty listed in Table 15.1) have the neutral R's. Two amino acids (aspartic and glutamic acids) have an extra carboxyl group and are acidic. T ...
... Amino acids can be classified as neutral, acidic, or basic, depending on the nature of their side chain, the R substituent. Most amino acids (fifteen of the twenty listed in Table 15.1) have the neutral R's. Two amino acids (aspartic and glutamic acids) have an extra carboxyl group and are acidic. T ...
CHEM 121 Chp 5 Spaulding
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
Acids and Bases
... Notice that water can behave as either an acid or a base. It can behave as an acid because it has a proton that it can donate, but it can also behave as a base because it has a lone pair that can accept a proton. In Section 2.2, we will see how we know that water acts as a base in the reaction on pa ...
... Notice that water can behave as either an acid or a base. It can behave as an acid because it has a proton that it can donate, but it can also behave as a base because it has a lone pair that can accept a proton. In Section 2.2, we will see how we know that water acts as a base in the reaction on pa ...
Chemistry 133 Problem Set Introduction
... 1.80 Until 1933, the United States minted gold coins for general circulation. The highest denomination produced was the twenty-dollar gold piece known as the double eagle. By an act of Congress in 1849, each double eagle weighed 516 grains and was 0.900 fine (33.436 g and 90.0 % gold (the remainder ...
... 1.80 Until 1933, the United States minted gold coins for general circulation. The highest denomination produced was the twenty-dollar gold piece known as the double eagle. By an act of Congress in 1849, each double eagle weighed 516 grains and was 0.900 fine (33.436 g and 90.0 % gold (the remainder ...
Chapter 14 - Chemistry Solutions
... • Ethers are unreactive toward base, but protonated ethers can undergo substitution reactions with strong acids. • Alcohol leaving group is replaced by a halide. • Reactivity: HI > HBr >> HCl ...
... • Ethers are unreactive toward base, but protonated ethers can undergo substitution reactions with strong acids. • Alcohol leaving group is replaced by a halide. • Reactivity: HI > HBr >> HCl ...
Chemistry of CHLORINE
... -Pale green colour of chlorine fades. b) Write two possible equations that take place. P4(s) + 6Cl2(g) → 4 PCl3(s) P4(s) + 10Cl2(g) → 4 PCl3(s) (c) State two reasons why the deflagrating spoon with rid/cover should be used. -Chlorine in the gas jar is poisonous/toxic. -Burning phosphorus produces po ...
... -Pale green colour of chlorine fades. b) Write two possible equations that take place. P4(s) + 6Cl2(g) → 4 PCl3(s) P4(s) + 10Cl2(g) → 4 PCl3(s) (c) State two reasons why the deflagrating spoon with rid/cover should be used. -Chlorine in the gas jar is poisonous/toxic. -Burning phosphorus produces po ...
8.1 Alcohols, Phenols, and Ethers 8.2 Naming Alcohols
... Methyl Alcohol (CH3OH, methanol) Methyl alcohol is the simplest (smallest) alcohol and is commonly known as wood alcohol because it was once prepared by heating wood in the absence of air. Ethyl Alcohol (CH3CH2OH, ethanol) Ethyl alcohol is one of the oldest known pure organic compounds. Ethyl alcoho ...
... Methyl Alcohol (CH3OH, methanol) Methyl alcohol is the simplest (smallest) alcohol and is commonly known as wood alcohol because it was once prepared by heating wood in the absence of air. Ethyl Alcohol (CH3CH2OH, ethanol) Ethyl alcohol is one of the oldest known pure organic compounds. Ethyl alcoho ...
amine
... nonbonded electron pair on the nitrogen atom, interconversion cannot occur, and the N atom is just like a carbon atom with four different groups around it. ...
... nonbonded electron pair on the nitrogen atom, interconversion cannot occur, and the N atom is just like a carbon atom with four different groups around it. ...
Unit 7: Reduction, Oxidation and Electrochemistry
... Examples: Na (s), O2 (g), O3 (g), H2 (g), F2 (g), P4 (s), and Hg (l) all have an oxidation number of 0. 2. Single Atomic Ions have an Oxidation Number Equals to its Charge. Example: K+ has an oxidation number of +1. 3. Oxygen in Binary Compound and Polyatomic Ions has an Oxidation Number of −2. Exam ...
... Examples: Na (s), O2 (g), O3 (g), H2 (g), F2 (g), P4 (s), and Hg (l) all have an oxidation number of 0. 2. Single Atomic Ions have an Oxidation Number Equals to its Charge. Example: K+ has an oxidation number of +1. 3. Oxygen in Binary Compound and Polyatomic Ions has an Oxidation Number of −2. Exam ...
PDF File
... ~ tenfold faster than that for the substrate with a 2′-fluoro group at U(–1), despite the weaker electron-withdrawing ability of 2′-OH than 2′-F [2]. As a 2′-fluoro group contains lone-pair electrons that can accept hydrogen bonds but cannot donate hydrogen bonds, the higher reactivity of the substr ...
... ~ tenfold faster than that for the substrate with a 2′-fluoro group at U(–1), despite the weaker electron-withdrawing ability of 2′-OH than 2′-F [2]. As a 2′-fluoro group contains lone-pair electrons that can accept hydrogen bonds but cannot donate hydrogen bonds, the higher reactivity of the substr ...
Advanced Practical Organic Chemistry
... expression CnH2n+2. The structural formula, shown for the first five alkanes in the table, shows each carbon atom and the elements that are attached to it. This structural formula is important when we begin to discuss more complex hydrocarbons. The simple alkanes share many properties in common. All ...
... expression CnH2n+2. The structural formula, shown for the first five alkanes in the table, shows each carbon atom and the elements that are attached to it. This structural formula is important when we begin to discuss more complex hydrocarbons. The simple alkanes share many properties in common. All ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... reactant, N2 and H2, available in a reaction. Thus, this is a limiting reactant problem. Plan If we assume that one reactant is completely consumed, we can calculate how much of the second reactant is needed in the reaction. By comparing the calculated quantity with the available amount, we can dete ...
... reactant, N2 and H2, available in a reaction. Thus, this is a limiting reactant problem. Plan If we assume that one reactant is completely consumed, we can calculate how much of the second reactant is needed in the reaction. By comparing the calculated quantity with the available amount, we can dete ...
4U Chemistry Practice Exam - Coristines
... d. 2A B 2C D B 2C D 2E F e. 2A C 2E F ____ 24. Which quantity does not increase when the temperature of a reaction system is raised? a. activation energy b. number of collisions c. number of effective collisions d. average kinetic energy of the particles e. all of the above inc ...
... d. 2A B 2C D B 2C D 2E F e. 2A C 2E F ____ 24. Which quantity does not increase when the temperature of a reaction system is raised? a. activation energy b. number of collisions c. number of effective collisions d. average kinetic energy of the particles e. all of the above inc ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... Ammonia is a weak base and is therefore only partially ionised in aqueous solution. Energy is required to ionise the ammonia molecules and hence less thermal energy is released. Any sensible value less negative than – 11.7. The accepted value is – 5.4 kJ mol−1. ...
... Ammonia is a weak base and is therefore only partially ionised in aqueous solution. Energy is required to ionise the ammonia molecules and hence less thermal energy is released. Any sensible value less negative than – 11.7. The accepted value is – 5.4 kJ mol−1. ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.