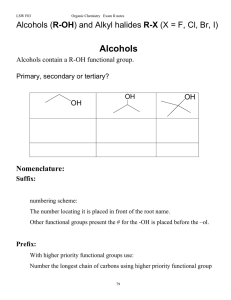
Alcohols (R-OH), and alkyl halides, RX
... More highly brominated by-products are possible if methyl bromide reacts with a bromine radical in the same fashion as methane does. Draw the cycle that leads to the formation of dibromomethane. ...
... More highly brominated by-products are possible if methyl bromide reacts with a bromine radical in the same fashion as methane does. Draw the cycle that leads to the formation of dibromomethane. ...
chemistry - Textbooks Online
... (i) The magnitude of order of a reaction may be zero, or fractional or integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reac ...
... (i) The magnitude of order of a reaction may be zero, or fractional or integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reac ...
Chapter 21: Organic Chemistry
... called cis-2-butene. In the structure on the right, the two methyl groups are on the opposite side of the double bond. Hence, this molecule is called trans-2-butene. Cis-2-butene and trans-2-butene are called “geometric isomers.” Geometric isomers often have different physical and chemical propertie ...
... called cis-2-butene. In the structure on the right, the two methyl groups are on the opposite side of the double bond. Hence, this molecule is called trans-2-butene. Cis-2-butene and trans-2-butene are called “geometric isomers.” Geometric isomers often have different physical and chemical propertie ...
Mark scheme - Unit F324 - Rings, polymers and analysis
... ALLOW a response that implies a splitting into three for a triplet/into four for a quartet Clear and unambiguous identification of the protons (when more than one type is present) other than by position number should be credited eg for CHNH2 could be HCCO or CHN or HCN or CH2CH eg for S-CH2-CH2 coul ...
... ALLOW a response that implies a splitting into three for a triplet/into four for a quartet Clear and unambiguous identification of the protons (when more than one type is present) other than by position number should be credited eg for CHNH2 could be HCCO or CHN or HCN or CH2CH eg for S-CH2-CH2 coul ...
Chapter 20 Organic Chemistry
... • Odorants must be volatile. • However, many volatile substances have no scent at all. • Most common smells are caused by organic molecules. • The study of compounds containing carbon combined with one or more of the elements hydrogen(H), nitrogen(N), oxygen(O), and sulfur(S), including their proper ...
... • Odorants must be volatile. • However, many volatile substances have no scent at all. • Most common smells are caused by organic molecules. • The study of compounds containing carbon combined with one or more of the elements hydrogen(H), nitrogen(N), oxygen(O), and sulfur(S), including their proper ...
Study Guide for Chapter 22 - Hydrocarbon Compounds
... 26.5°C. The water reached a maximum temperature of 27.3°C. How many joules of heat were released by the silver dollar? (Chapter 17 ) 83. What is the relationship between a calorie and a ...
... 26.5°C. The water reached a maximum temperature of 27.3°C. How many joules of heat were released by the silver dollar? (Chapter 17 ) 83. What is the relationship between a calorie and a ...
Chapter 14: Chemical Equilibrium
... Predicting the Direction of Change: Q vs K compare Q and K if Q = K: the system is at equilibrium if Q < K: the system is not at equilibrium the reaction proceeds in the forward direction to reach equilibrium if Q > K: the system is not at equilibrium the reaction proceeds in the reverse direction t ...
... Predicting the Direction of Change: Q vs K compare Q and K if Q = K: the system is at equilibrium if Q < K: the system is not at equilibrium the reaction proceeds in the forward direction to reach equilibrium if Q > K: the system is not at equilibrium the reaction proceeds in the reverse direction t ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... Definitions for vocabulary words can be found in the Illustrated Glossary of Organic Chemistry, available at the course web site. Discussion: Every organic compound needs an unambiguous name that clearly delineates all structural features of the molecule. The same is true for stereocenters. Because ...
... Definitions for vocabulary words can be found in the Illustrated Glossary of Organic Chemistry, available at the course web site. Discussion: Every organic compound needs an unambiguous name that clearly delineates all structural features of the molecule. The same is true for stereocenters. Because ...
Reactions of carbon radicals generated by 1,5
... lead(IV) alkoxides 9. Transposition of the radical center, i.e., an internal 1,5-hydrogen transfer, is a characteristic reaction of alkoxy radicals 10, resulting in the generation of the corresponding d-carbon radicals 11. The carbon radical 11, however, exists as a tight radical pair with its •Pb(O ...
... lead(IV) alkoxides 9. Transposition of the radical center, i.e., an internal 1,5-hydrogen transfer, is a characteristic reaction of alkoxy radicals 10, resulting in the generation of the corresponding d-carbon radicals 11. The carbon radical 11, however, exists as a tight radical pair with its •Pb(O ...
A Model For the Calculation of Solvent ... Reaction Rates for Process Design Purposes
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
VITA - Trace: Tennessee Research and Creative Exchange
... that strong σ-donor mono-, bi-, and tridentate ligands can be used to stabilize the metal ligand multiple bond (Figure 1.1A-C, respectively).10 In one example by Peters, it is found that the iron trisphosphinoborate complex (Figure 1.1C) can even activate an organic azide, however, the resulting imi ...
... that strong σ-donor mono-, bi-, and tridentate ligands can be used to stabilize the metal ligand multiple bond (Figure 1.1A-C, respectively).10 In one example by Peters, it is found that the iron trisphosphinoborate complex (Figure 1.1C) can even activate an organic azide, however, the resulting imi ...
Chapter 1
... • Typical saturated fatty acids are tightly packed together • cis double bonds prevent good alignment of molecules in unsaturated fatty acids leading to poor packing • Double bonds lower melting point relative to saturated acid ...
... • Typical saturated fatty acids are tightly packed together • cis double bonds prevent good alignment of molecules in unsaturated fatty acids leading to poor packing • Double bonds lower melting point relative to saturated acid ...
Chapter 1 – Reaction Kinetics Answer Key
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
Magnesium - Cometal S.A.
... Magnesium is chemically very active, and displaces hydrogen in boiling water and a large number of metals can be prepared by thermal reduction of its salts and oxides with magnesium. It combines with most non-metals and virtually all acids. Magnesium does not or only slightly react with most of the ...
... Magnesium is chemically very active, and displaces hydrogen in boiling water and a large number of metals can be prepared by thermal reduction of its salts and oxides with magnesium. It combines with most non-metals and virtually all acids. Magnesium does not or only slightly react with most of the ...
2007 Nov Paper 1 - A Level Tuition
... maximum safe pressure difference between the air inside the tyre and the atmospheric pressure acting on it = 8 – 1.01 = 6.99 bar pressure difference across the wall of the tyre in flight = 5.69 – 0.28 = 5.41 bar Hence, it is not necessary to reduce the air pressure inside the tyre since difference b ...
... maximum safe pressure difference between the air inside the tyre and the atmospheric pressure acting on it = 8 – 1.01 = 6.99 bar pressure difference across the wall of the tyre in flight = 5.69 – 0.28 = 5.41 bar Hence, it is not necessary to reduce the air pressure inside the tyre since difference b ...
HOTS Worksheet
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
File - cpprashanths Chemistry
... Q18. What is an antibiotic? Name the first antibiotic discovered.Is this antibiotic narrow spectrum or broad spectrum antibiotic? Antibiotics are chemical substances which are produced by microorganisms inorder to inhibit the growth or even to destroy other microorganisms. 1M The first antibiotic di ...
... Q18. What is an antibiotic? Name the first antibiotic discovered.Is this antibiotic narrow spectrum or broad spectrum antibiotic? Antibiotics are chemical substances which are produced by microorganisms inorder to inhibit the growth or even to destroy other microorganisms. 1M The first antibiotic di ...
Mole Concept - Shailendra Kumar Chemistry
... One of the earliest method for determining the molecular weight of protein was based on chemical analysis. A haemoglobin preparation was found to contain 0.335% iron. (a) If the haemoglobin molecule contain one atom of iron. What is its molecular weight ? (b) If the haemoglobin molecule contains fou ...
... One of the earliest method for determining the molecular weight of protein was based on chemical analysis. A haemoglobin preparation was found to contain 0.335% iron. (a) If the haemoglobin molecule contain one atom of iron. What is its molecular weight ? (b) If the haemoglobin molecule contains fou ...
Alcohols Phenols and Ethers
... 2. With unsaturated alcohols, two endings are needed, one for the double or triple bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydrox ...
... 2. With unsaturated alcohols, two endings are needed, one for the double or triple bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydrox ...
13: Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids
... Details of Oxidation Number Calculations. The rules used to calculate these numbers are similar to those used for inorganic molecules. The H atoms are assigned an oxidation number of +1 when they are bonded to C or to more electronegative atoms. In contrast, oxygen atoms, whether singly or doubly bo ...
... Details of Oxidation Number Calculations. The rules used to calculate these numbers are similar to those used for inorganic molecules. The H atoms are assigned an oxidation number of +1 when they are bonded to C or to more electronegative atoms. In contrast, oxygen atoms, whether singly or doubly bo ...
chemistry - Ethiopian Ministry of Education
... Since chemistry is such an enormous area of science, for convenience it has been divided into disciplines. However, the division is never as clear-cut as it might appear to be. All sciences are related and depend on each other – they are interrelated. All the disciplines of science share information ...
... Since chemistry is such an enormous area of science, for convenience it has been divided into disciplines. However, the division is never as clear-cut as it might appear to be. All sciences are related and depend on each other – they are interrelated. All the disciplines of science share information ...
ch221 class 5
... Many compounds are still preferentially given trivial names, examples including formic acid, derived from ants (Latin: Formica) and salicylic acid, which was first discovered as a component of the bark of willow trees (Latin: Salix). As more and more complex organic compounds were discovered, the n ...
... Many compounds are still preferentially given trivial names, examples including formic acid, derived from ants (Latin: Formica) and salicylic acid, which was first discovered as a component of the bark of willow trees (Latin: Salix). As more and more complex organic compounds were discovered, the n ...
chemistry - Brilliant Public School Sitamarhi
... *12. A compound made up of elements ‘A’ and ‘B’ crystallises in a cubic close packed structure. Atoms A are present on the corners as well as face centres, whereas atoms B are present on the edge-centres as well as body centre. What is the formula of the compound? [Ans. AB] ...
... *12. A compound made up of elements ‘A’ and ‘B’ crystallises in a cubic close packed structure. Atoms A are present on the corners as well as face centres, whereas atoms B are present on the edge-centres as well as body centre. What is the formula of the compound? [Ans. AB] ...
here
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.