Alberta Chemistry 20-30 Sample CAB Questions - McGraw
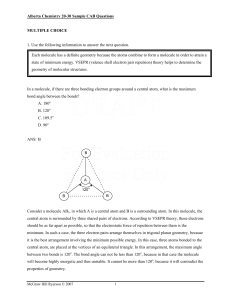
... should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geometry, because it is the best arrangement involving the minimum possible energy. In this case, three atoms b ...
... should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geometry, because it is the best arrangement involving the minimum possible energy. In this case, three atoms b ...
Appendix
... These basic anhydrides form hydroxides in water. Example: K2O(s) + H2O(l) ⟶ 2KOH(aq) Li, Na, Rb, and Cs also follow this pattern. ...
... These basic anhydrides form hydroxides in water. Example: K2O(s) + H2O(l) ⟶ 2KOH(aq) Li, Na, Rb, and Cs also follow this pattern. ...
Oxygen Carriers Materials for Chemical
... from combustion air to the fuel. Two inter-connected fluidized beds, a fuel and an air reactor, are used in the process. Whereas the natural gas is fully oxidized to CO2 and H2O in the fuel reactor for CLC, it is only partially oxidized by the metal oxide in the fuel reactor for CLR, resulting in a ...
... from combustion air to the fuel. Two inter-connected fluidized beds, a fuel and an air reactor, are used in the process. Whereas the natural gas is fully oxidized to CO2 and H2O in the fuel reactor for CLC, it is only partially oxidized by the metal oxide in the fuel reactor for CLR, resulting in a ...
Atmospheric Formation_TELTEK
... contained ca. 3 µg m-3 (1.6 ppb) CHONH2 (formamide) and another amide-like compound with M=87 was detected but not identified and quantified. Schade and Crutzen17 suggested that this mass could correspond to CHO-N(CH3)-CHO (N-formyl, N-methylformamide). Murphy et al.18 reported that the aerosol whic ...
... contained ca. 3 µg m-3 (1.6 ppb) CHONH2 (formamide) and another amide-like compound with M=87 was detected but not identified and quantified. Schade and Crutzen17 suggested that this mass could correspond to CHO-N(CH3)-CHO (N-formyl, N-methylformamide). Murphy et al.18 reported that the aerosol whic ...
Chapter 6
... expedition to Egypt. While visiting the Natron Lakes, a series of salt water lakes carved from limestone, Berthollet made an observation that led him to an important discovery. When exploring the lake’s shore Berthollet found deposits of Na2CO3, a result he found surprising. Why did Berthollet find ...
... expedition to Egypt. While visiting the Natron Lakes, a series of salt water lakes carved from limestone, Berthollet made an observation that led him to an important discovery. When exploring the lake’s shore Berthollet found deposits of Na2CO3, a result he found surprising. Why did Berthollet find ...
Carbocation Stability
... The more stable the carbocation, the faster it is formed. Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
... The more stable the carbocation, the faster it is formed. Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
tyrosinase in crustacean blood - Journal of Experimental Biology
... formed. This is due partly to the fact that the enzyme is less active at this low hydrogen-ion concentration, and partly because oxidation of the red quinone to the later products of the reaction proceeds more rapidly in alkaline solutions. Under these conditions, the first visible sign of the oxida ...
... formed. This is due partly to the fact that the enzyme is less active at this low hydrogen-ion concentration, and partly because oxidation of the red quinone to the later products of the reaction proceeds more rapidly in alkaline solutions. Under these conditions, the first visible sign of the oxida ...
Unit 4 - Chemical Equilibrium
... N2 ( g ) + 3 H2 (g) 2 NH3 ( g ) increase conc. of N2 -----> stress too much to relieve stress equilibrium shifts to the ; thus conc. of H2 will and conc. of NH3 will ____________ Consider the equ. conc to be N2 = 1.0 moL/L; H2 = 3.0 moL/L and NH3 = 2.0 moL/L If an additional 0.5 moL/L of N2 is add ...
... N2 ( g ) + 3 H2 (g) 2 NH3 ( g ) increase conc. of N2 -----> stress too much to relieve stress equilibrium shifts to the ; thus conc. of H2 will and conc. of NH3 will ____________ Consider the equ. conc to be N2 = 1.0 moL/L; H2 = 3.0 moL/L and NH3 = 2.0 moL/L If an additional 0.5 moL/L of N2 is add ...
organic chemistry - Sakshieducation.com
... 3. Explain the structure and chemical nature of ethers. Ans: Structure : In CH3OCH3, the central oxygen atom is sp3 hybridized with two completely filled sp3 orbitals having lone pair of electrons and two half filled sp3 hybridized orbitals. Also carbon atoms are sp3 hybridized and both the half fil ...
... 3. Explain the structure and chemical nature of ethers. Ans: Structure : In CH3OCH3, the central oxygen atom is sp3 hybridized with two completely filled sp3 orbitals having lone pair of electrons and two half filled sp3 hybridized orbitals. Also carbon atoms are sp3 hybridized and both the half fil ...
Laboratory Manual
... 6. Safety Goggles – Safety goggles are worn to protect your eyes. They should be worn at all times when you are in the chemistry laboratory. Even if you are not working with chemicals, someone else might be. They could splash a chemical in your eyes. 7. Gloves – At times you may need to wear gloves ...
... 6. Safety Goggles – Safety goggles are worn to protect your eyes. They should be worn at all times when you are in the chemistry laboratory. Even if you are not working with chemicals, someone else might be. They could splash a chemical in your eyes. 7. Gloves – At times you may need to wear gloves ...
Post Lab Questions
... Backpacks and bags are not allowed to sit on the floor. Hooks are conveniently placed on desks to hang your backpacks and bags. During the period Do not ask to leave the class for personal reasons. Please remain in your seat during class time unless instructed otherwise. Raise your hand if you wish ...
... Backpacks and bags are not allowed to sit on the floor. Hooks are conveniently placed on desks to hang your backpacks and bags. During the period Do not ask to leave the class for personal reasons. Please remain in your seat during class time unless instructed otherwise. Raise your hand if you wish ...
Answer Key - mrkelleher
... c. The second heating is to ensure that all the water in the sample has been driven off. If the mass is less after the second heating, water was still present after the first |heating. 5. a. CF2 b. C4F8 6. a. CuClO3 b. copper(I) chlorate ...
... c. The second heating is to ensure that all the water in the sample has been driven off. If the mass is less after the second heating, water was still present after the first |heating. 5. a. CF2 b. C4F8 6. a. CuClO3 b. copper(I) chlorate ...
Regents Chemistry Review - New York Science Teacher
... The table gives information about the nucleus of each of four atoms. • How many different elements are represented by the nuclei in the table? ...
... The table gives information about the nucleus of each of four atoms. • How many different elements are represented by the nuclei in the table? ...
85 Q.2 Pure water has a low electricity conductivity because A. it
... D. a layer of oxide film is formed on the surface of zinc to prevent further reaction. 90 Q.22 X is white solid. When dilute hydrochloric acid is added to X, a colourless gas is liberated. An aqueous solution of X gives a white precipitate with silver nitrate solution. X is probably A. C. ...
... D. a layer of oxide film is formed on the surface of zinc to prevent further reaction. 90 Q.22 X is white solid. When dilute hydrochloric acid is added to X, a colourless gas is liberated. An aqueous solution of X gives a white precipitate with silver nitrate solution. X is probably A. C. ...
Project Overview
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
... Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Gu ...
Exam #3
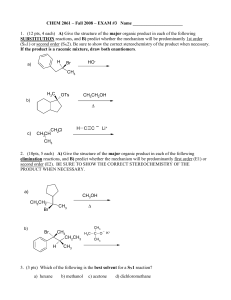
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
Chapter 4
... We begin by studying the properties of solutions prepared by dissolving substances in water, called aqueous solutions. Aqueous solutions can be classified as nonelectrolyte or electrolyte, depending on their ability to conduct electricity. (4.1) ...
... We begin by studying the properties of solutions prepared by dissolving substances in water, called aqueous solutions. Aqueous solutions can be classified as nonelectrolyte or electrolyte, depending on their ability to conduct electricity. (4.1) ...
HEAd START TO A LEVEL CHEMISTRY WORKbOOK
... A molecule is the smallest, electrically neutral, particle of an element or compound that can exist on its own. An ion is an atom, or group of atoms, which carries an electric charge. You need to know these definitions by heart, but you also need to be able to recognise the formulae of atoms and mol ...
... A molecule is the smallest, electrically neutral, particle of an element or compound that can exist on its own. An ion is an atom, or group of atoms, which carries an electric charge. You need to know these definitions by heart, but you also need to be able to recognise the formulae of atoms and mol ...
Module 1 Predictor Questions
... converting one unit to another. Note that each unit factor may be written in two equivalent ways. The one you use depends on what units you are trying to cancel in a dimensional analysis problem (see examples below). One way to help insure that you work conversion problems correctly is to remember w ...
... converting one unit to another. Note that each unit factor may be written in two equivalent ways. The one you use depends on what units you are trying to cancel in a dimensional analysis problem (see examples below). One way to help insure that you work conversion problems correctly is to remember w ...
Organic Chemistry I-2 Ans Chapter 7 Free Radical Answers 1
... 4. What nucleophiles could be used to react with butyl bromide to prepare the following compounds? a. HOB. CH3CH2SH C. CH3OH D. CH3NH2 E. CN- F. NaSH 5. Which one in each of the following pairs will react faster in an SN2 reaction with OH-? a. CH3I (better leaving group) b. dimethyl sulfoxide will n ...
... 4. What nucleophiles could be used to react with butyl bromide to prepare the following compounds? a. HOB. CH3CH2SH C. CH3OH D. CH3NH2 E. CN- F. NaSH 5. Which one in each of the following pairs will react faster in an SN2 reaction with OH-? a. CH3I (better leaving group) b. dimethyl sulfoxide will n ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.