Perfumery_Synthetics_and_Isolates_pg_283
... linalyl acetate and may contain this ester to the extent of over 40 per cent. The delicate and highly pleasant odor of linalool and its esters plays an important part in the composition of many floral perfumes, colognes, and various other formulations. Because of the relatively low boiling point, li ...
... linalyl acetate and may contain this ester to the extent of over 40 per cent. The delicate and highly pleasant odor of linalool and its esters plays an important part in the composition of many floral perfumes, colognes, and various other formulations. Because of the relatively low boiling point, li ...
Compounds with Oxygen Atoms
... • in the IUPAC system replace the -e with -ol. • with common names use the name of the alkyl group followed by alcohol. ...
... • in the IUPAC system replace the -e with -ol. • with common names use the name of the alkyl group followed by alcohol. ...
- University of Bath Opus
... borrowing hydrogen methodology with masked ammonia sources. This unique approach allows selective access to primary amines without the over alkylation seen when using other ammonia sources. Furthermore, the borrowing hydrogen reaction can be coupled with an in-situ palladium catalysed hydrogenolysis ...
... borrowing hydrogen methodology with masked ammonia sources. This unique approach allows selective access to primary amines without the over alkylation seen when using other ammonia sources. Furthermore, the borrowing hydrogen reaction can be coupled with an in-situ palladium catalysed hydrogenolysis ...
Equilibrium Reversible Reactions
... You start at the top (reactants) and end up at the bottom (products). But when you are partway down you start walking up the escalator as it continues going down. If you match your rate of w ...
... You start at the top (reactants) and end up at the bottom (products). But when you are partway down you start walking up the escalator as it continues going down. If you match your rate of w ...
Hazards and Precautions FORMALDEHIDE Formaldehyde
... concentrations, is a combustible liquid. In addition, formaldehyde reacts with hydrochloric acid (HCl) to produce bis (chloromethyl) ether vapor, a very potent carcinogen. ...
... concentrations, is a combustible liquid. In addition, formaldehyde reacts with hydrochloric acid (HCl) to produce bis (chloromethyl) ether vapor, a very potent carcinogen. ...
2013 - SQA
... provided in this answer book, and must be written clearly and legibly in ink. 3 Rough work, if any should be necessary, should be written in this book and then scored through when the fair copy has been written. If further space is required, a supplementary sheet for rough work may be obtained from ...
... provided in this answer book, and must be written clearly and legibly in ink. 3 Rough work, if any should be necessary, should be written in this book and then scored through when the fair copy has been written. If further space is required, a supplementary sheet for rough work may be obtained from ...
Chapter 19 Carboxylic Acid Derivatives Nucleophilic Acyl
... For thioesters: Sulfur is a third row element, like chlorine, and so it is considerably larger than oxygen. The C-S bond is relatively long and this makes for poor overlap of the lone pair 3p orbital and the π-orbital of the carbonyl. But thioesters are less reactive than acid chlorides and anhydrid ...
... For thioesters: Sulfur is a third row element, like chlorine, and so it is considerably larger than oxygen. The C-S bond is relatively long and this makes for poor overlap of the lone pair 3p orbital and the π-orbital of the carbonyl. But thioesters are less reactive than acid chlorides and anhydrid ...
102MSJc14 - Louisiana Tech University
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
... Any chemical reaction could be considered as a forward and backward reactions occurring at the same time( ) as described previously. If the rates of backward and forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
the chemical and physical properties of condensed
... Examples of the influence of external factors upon the transitions of phosphates are found in the transitions of sodium tetrametaphosphate tetrahydrate and sodium triphosphate hexahydrate. The tetrametaphosphate will be discussed first. In this transition Na4P4O124H2O suffers a crystallographic chan ...
... Examples of the influence of external factors upon the transitions of phosphates are found in the transitions of sodium tetrametaphosphate tetrahydrate and sodium triphosphate hexahydrate. The tetrametaphosphate will be discussed first. In this transition Na4P4O124H2O suffers a crystallographic chan ...
ALCOHOLS, ETHERS, PHENOLS, AND THIOLS
... CH 3 ¬ C “ CH ¬ CH 3 Hydration will not form 2-methyl- l-butanol because the double bond lies between the middle two carbons in 2-methyl-2-butene. By Markovnikov’s Rule, the ¬ H will add to the double bonded carbon that already has a hydrogen. Thus, 2-methyl2-butanol will be the major product. ...
... CH 3 ¬ C “ CH ¬ CH 3 Hydration will not form 2-methyl- l-butanol because the double bond lies between the middle two carbons in 2-methyl-2-butene. By Markovnikov’s Rule, the ¬ H will add to the double bonded carbon that already has a hydrogen. Thus, 2-methyl2-butanol will be the major product. ...
Modern Synthetic Methods for Copper-Mediated C(aryl
... that these new conditions were ideal to effect an Ullmann condensation. At this point, there was little deliberation on the mechanistic rationale for these precise reaction conditions, although the outcome is of considerable importance. Chan and co-workers[18] first described a collection of Nand O- ...
... that these new conditions were ideal to effect an Ullmann condensation. At this point, there was little deliberation on the mechanistic rationale for these precise reaction conditions, although the outcome is of considerable importance. Chan and co-workers[18] first described a collection of Nand O- ...
CH221 CLASS 13
... Treatment of alkenes with mercury (II) acetate in aqueous tetrahydrofuran (THF), followed by reduction with sodium borohydride (NaBH4), leads to alcohols. The Overall addition is Markovnikov in orientation (regioselectivity), as illustrated by the example below. ...
... Treatment of alkenes with mercury (II) acetate in aqueous tetrahydrofuran (THF), followed by reduction with sodium borohydride (NaBH4), leads to alcohols. The Overall addition is Markovnikov in orientation (regioselectivity), as illustrated by the example below. ...
contact - DTU Kemi
... ephedrine – all “household names” – are members of the alkaloid family, a group of naturally occurring compounds containing nitrogen atoms. Besides these well-known examples, a large variety of other alkaloids exist. Many of these occur in nature, while others are synthetic, typically produced as dr ...
... ephedrine – all “household names” – are members of the alkaloid family, a group of naturally occurring compounds containing nitrogen atoms. Besides these well-known examples, a large variety of other alkaloids exist. Many of these occur in nature, while others are synthetic, typically produced as dr ...
chem 102 class notes - Louisiana Tech University
... Any chemical reaction could be considered as a forward and backward reactions ) as described previously. If the rates of backward and occurring at the same time( forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
... Any chemical reaction could be considered as a forward and backward reactions ) as described previously. If the rates of backward and occurring at the same time( forward reactions chemical reactions are comparable both reactants and products can coexist leading to a condition called chemical equilib ...
Here
... An organic compound consists of molecules made from carbon, hydrogen and oxygen atoms. There can be other atoms – for example, nitrogen, sulfur. 1. Acetylsalicylic acid, the common mild pain reliever, is a derivative of the natural product, salicin, obtained from willow bark. 2. Morphine, obt ...
... An organic compound consists of molecules made from carbon, hydrogen and oxygen atoms. There can be other atoms – for example, nitrogen, sulfur. 1. Acetylsalicylic acid, the common mild pain reliever, is a derivative of the natural product, salicin, obtained from willow bark. 2. Morphine, obt ...
Post Lab Questions
... Do not place hot apparatuses directly on the laboratory desk. Always use an insulating pad. Allow plenty of time for hot apparatuses to cool before touching. When bending glass, allow time for the glass to cool before handling. Hot and cold glass have the same visual appearance. Determine if an obje ...
... Do not place hot apparatuses directly on the laboratory desk. Always use an insulating pad. Allow plenty of time for hot apparatuses to cool before touching. When bending glass, allow time for the glass to cool before handling. Hot and cold glass have the same visual appearance. Determine if an obje ...
CHEM 494 Lecture 5 - UIC Department of Chemistry
... activation energy; positive and negative atoms bond fast • products are much lower in energy since they are neutral; exothermic reaction ...
... activation energy; positive and negative atoms bond fast • products are much lower in energy since they are neutral; exothermic reaction ...
Chemistry 0310 - Organic Chemistry 1 Chapter 12. Reactions of
... - Syn-Hydroxylations of alkenes are most conveniently performed with catalytic OsO4 and NMO (N-methylmorpholine N-oxide) as co-oxidant. Attack occurs from the less-hindered face of the alkene, and a vicinal syn-diol is isolated after reductive workup. - Oxidative cleavage of 1,2-disubstituted alken ...
... - Syn-Hydroxylations of alkenes are most conveniently performed with catalytic OsO4 and NMO (N-methylmorpholine N-oxide) as co-oxidant. Attack occurs from the less-hindered face of the alkene, and a vicinal syn-diol is isolated after reductive workup. - Oxidative cleavage of 1,2-disubstituted alken ...
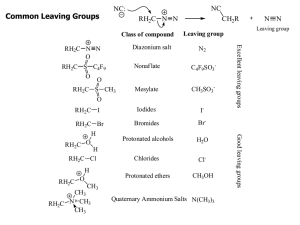
Common Leaving Groups
... C atoms with more s character tend to form stronger bonds with other carbons. R ...
... C atoms with more s character tend to form stronger bonds with other carbons. R ...
中国科学技术大学有机合成讲义-2
... - DMP has found wide utility in the preparation of sensitive, highly functionalized molecules. DMP oxidations are characterized by short reaction times, use of a single equivalent of oxidant, and can be moderated with regard to acidity by the incorporation of additives such as pyridine. DMP and its ...
... - DMP has found wide utility in the preparation of sensitive, highly functionalized molecules. DMP oxidations are characterized by short reaction times, use of a single equivalent of oxidant, and can be moderated with regard to acidity by the incorporation of additives such as pyridine. DMP and its ...
1.4. Nomenclature 2. LABORATORY
... 14 per cent concentration) and z mol of sulphuric acid of density 1-84 containing 85-2.0 per cent of copper salt at 145-150°, ethylene was obtained in 15-20 per cent 1c4dand 99 per cent purity. According to Scnderens4 the addition of aluminium ~lphateincreascs the rate of production of ethylene, but ...
... 14 per cent concentration) and z mol of sulphuric acid of density 1-84 containing 85-2.0 per cent of copper salt at 145-150°, ethylene was obtained in 15-20 per cent 1c4dand 99 per cent purity. According to Scnderens4 the addition of aluminium ~lphateincreascs the rate of production of ethylene, but ...
Wafer-Level Artificial Photosynthesis for CO2 Reduction into CH4
... bond C=O can be broken to form C-H bonds, which leads to the formation of different fuels such as methane (CH4). H2 formation is a very competitive process in CO2 photochemical and photoelectrochemical reduction. This is due to the use of water as an atomic hydrogen donor for CO2 reduction, whereby ...
... bond C=O can be broken to form C-H bonds, which leads to the formation of different fuels such as methane (CH4). H2 formation is a very competitive process in CO2 photochemical and photoelectrochemical reduction. This is due to the use of water as an atomic hydrogen donor for CO2 reduction, whereby ...
Nitrogen and Oxygen Family
... (c) Catalyst : At low temperature, although the yield of ammonia is more yet the reaction is very slow. In order to speed up the reaction, a catalyst is used. The following catalysts have been proposed for this purpose. (i) Finely divided iron with some molybdenum as a promotor. (ii) Finely divided ...
... (c) Catalyst : At low temperature, although the yield of ammonia is more yet the reaction is very slow. In order to speed up the reaction, a catalyst is used. The following catalysts have been proposed for this purpose. (i) Finely divided iron with some molybdenum as a promotor. (ii) Finely divided ...
Rubidium
... It occurs naturally in the minerals leucite, pollucite, and zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially signif ...
... It occurs naturally in the minerals leucite, pollucite, and zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially signif ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.