LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 27. Bromination of an organic compound(A) of formula C6H12 yields C6H12Br2. On reduction of the compound gives 2-methylpentane. On oxidation it yields a mixture of acetic acid and isobutyric acids. Assign the structural formula to the organic compound. Express the above reaction by chemical equation ...
... 27. Bromination of an organic compound(A) of formula C6H12 yields C6H12Br2. On reduction of the compound gives 2-methylpentane. On oxidation it yields a mixture of acetic acid and isobutyric acids. Assign the structural formula to the organic compound. Express the above reaction by chemical equation ...
Chapter 4 - The Study of Chemical Reactions
... 1. Sect. 4.3 Transition state for electrophilic addition to an alkene A useful method to approximate a transition state structure is to examine the reactant and product, note which bonds must be broken and/or made, and then write a state with partial bond making and/or breaking, sort of halfway in b ...
... 1. Sect. 4.3 Transition state for electrophilic addition to an alkene A useful method to approximate a transition state structure is to examine the reactant and product, note which bonds must be broken and/or made, and then write a state with partial bond making and/or breaking, sort of halfway in b ...
Conventional Catalytic cycle for hydrogenation with Wilkinson`s
... having a CH2OH side group. By virtue of the hydroxyl group, serine is classified as a polar amino acid. Serine was first obtained from silk protein, a particularly rich source, in ...
... having a CH2OH side group. By virtue of the hydroxyl group, serine is classified as a polar amino acid. Serine was first obtained from silk protein, a particularly rich source, in ...
lecture 11 catalysis_hydrogenation of alkenes
... dihydride leads to labilization of one of the PPh3 ligands (high trans effect of H) to give a site at which the alkene binds. ...
... dihydride leads to labilization of one of the PPh3 ligands (high trans effect of H) to give a site at which the alkene binds. ...
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
Reductive etherification of substituted cyclohexanones with
... by the solvent, can be excluded, since no reaction occurred on starting from 4-tert-butylcyclohexanol under similar reaction conditions. It seems more likely that the reaction proceeds via Brønsted acid-catalysed formation of a hemiacetal followed by a Lewis acid-catalysed, MPV-like hydride transfer ...
... by the solvent, can be excluded, since no reaction occurred on starting from 4-tert-butylcyclohexanol under similar reaction conditions. It seems more likely that the reaction proceeds via Brønsted acid-catalysed formation of a hemiacetal followed by a Lewis acid-catalysed, MPV-like hydride transfer ...
Final Exam Review Sheet Chemistry 110a/1998
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
Chapter 17 Aldehydes and Ketones
... • This reagent contains hydrogen in the form of hydride ion, H:-. • In a reduction by sodium borohydride, hydride ion adds to the partially positive carbonyl carbon which leaves a negative charge on the carbonyl oxygen. • Reaction of this intermediate with aqueous acid gives the alcohol. ...
... • This reagent contains hydrogen in the form of hydride ion, H:-. • In a reduction by sodium borohydride, hydride ion adds to the partially positive carbonyl carbon which leaves a negative charge on the carbonyl oxygen. • Reaction of this intermediate with aqueous acid gives the alcohol. ...
review sheet plus practice problems
... text. The test will focus on chemistry of alkyl halides and alcohols, and organic synthesis. The following textbook sections will be covered on the exam: 10.1 to 10.7, 10.10 ...
... text. The test will focus on chemistry of alkyl halides and alcohols, and organic synthesis. The following textbook sections will be covered on the exam: 10.1 to 10.7, 10.10 ...
Ch 19 test_take-home
... A) the reverse process is spontaneous but the forward process is not B) the forward and the reverse processes are both spontaneous C) the forward process is spontaneous but the reverse process is not D) the process is not spontaneous in either direction E) both forward and reverse processes have sto ...
... A) the reverse process is spontaneous but the forward process is not B) the forward and the reverse processes are both spontaneous C) the forward process is spontaneous but the reverse process is not D) the process is not spontaneous in either direction E) both forward and reverse processes have sto ...
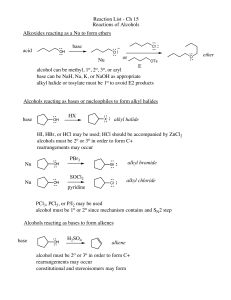
Chapter Nine: Alcohols, Ethers and Epoxides
... a. Synthesis from halohydrins in base: internal SN2 review b. Reactivity: 9.15 i. Cleavage in Acid: “SN1 like”: Nucleophile to more substituted carbon with inversion (examples: HBr, H3O+, CH3OH2+) ii. Cleavage in Base: “SN2 like” Nucleophile to less crowded carbon with inversion (examples: CH3NH2, C ...
... a. Synthesis from halohydrins in base: internal SN2 review b. Reactivity: 9.15 i. Cleavage in Acid: “SN1 like”: Nucleophile to more substituted carbon with inversion (examples: HBr, H3O+, CH3OH2+) ii. Cleavage in Base: “SN2 like” Nucleophile to less crowded carbon with inversion (examples: CH3NH2, C ...
C h e m g u id e –... ESTERS: PREPARATION
... d) The ester phenyl benzoate can be made from a reaction starting from benzoyl chloride, C6H5COCl. However, in this case, the phenol is first converted into sodium phenoxide, C6H5O- Na+. (i) Why is it necessary to use sodium phenoxide rather than phenol itself? (ii) How do you convert phenol into so ...
... d) The ester phenyl benzoate can be made from a reaction starting from benzoyl chloride, C6H5COCl. However, in this case, the phenol is first converted into sodium phenoxide, C6H5O- Na+. (i) Why is it necessary to use sodium phenoxide rather than phenol itself? (ii) How do you convert phenol into so ...
Aldehydes and Ketones Both contain the functional group C O
... CH 3CH2CH2CH2CH 2OH NaHSO3/H2 O extract with ether ...
... CH 3CH2CH2CH2CH 2OH NaHSO3/H2 O extract with ether ...
IGCSE Chemistry Definitions – LEARN THESE! Melting
... Eutrophication - Process where lakes and rivers which are rich in nutrients due to leaching of fertilizers, encourages the growth of plant life which is decomposed by bacteria using oxygen in the water. Crude Oil - A mixture of hydrocarbons formed from the remains of dead sea life which was covered ...
... Eutrophication - Process where lakes and rivers which are rich in nutrients due to leaching of fertilizers, encourages the growth of plant life which is decomposed by bacteria using oxygen in the water. Crude Oil - A mixture of hydrocarbons formed from the remains of dead sea life which was covered ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.