Microsoft Word
... higher than ambient, and other advantages. 1 Unlike ionic reaction intermediates, however, the growing radical species therein usually suffers from bimolecular termination reactions such as radical recombination and disproportionation. So, the radical polymerization technique is considered unsuitabl ...
... higher than ambient, and other advantages. 1 Unlike ionic reaction intermediates, however, the growing radical species therein usually suffers from bimolecular termination reactions such as radical recombination and disproportionation. So, the radical polymerization technique is considered unsuitabl ...
Theoretical Study of Gas-Phase Reactions of Fe(CO)5 with OH
... explored for the last stages of this chemical process as a mechanistic alternative to regenerate the starting catalyst. Introduction The water gas shift reaction (WGSR), eq 1, is a key process in the worldwide chemical industry.1 Its importance derives from its role both as a means for enriching the ...
... explored for the last stages of this chemical process as a mechanistic alternative to regenerate the starting catalyst. Introduction The water gas shift reaction (WGSR), eq 1, is a key process in the worldwide chemical industry.1 Its importance derives from its role both as a means for enriching the ...
- kunleoloruntegbe.com
... Alchohol addition: Alkanals, but not alkanones, will give addition reactions with alcohols provided all the reagent are dry, and that Hydrochloric acid (HCL) is used to catalyse the reaction. The most common example of this type of addition is ethanol additing to ethanal. dry CH3CHO + 2C2 H5 OH HCl ...
... Alchohol addition: Alkanals, but not alkanones, will give addition reactions with alcohols provided all the reagent are dry, and that Hydrochloric acid (HCL) is used to catalyse the reaction. The most common example of this type of addition is ethanol additing to ethanal. dry CH3CHO + 2C2 H5 OH HCl ...
Nomenclature of Organic Compounds
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
Displacement Reactions Between Metal Ions and Nitride Barrier
... palladous complex. In addition, the chemical shift in this peak reveals that the original complex bonding might have been interfered with or even replaced by other generated species, e.g., titanium halide. Moreover, BHF was replaced by NaF to eliminate the possible interference from ammonium. The re ...
... palladous complex. In addition, the chemical shift in this peak reveals that the original complex bonding might have been interfered with or even replaced by other generated species, e.g., titanium halide. Moreover, BHF was replaced by NaF to eliminate the possible interference from ammonium. The re ...
Document
... o average energy (dashed line) of substrates (higher on graph) and products (lower on graph) o delta G starts at substrate line and goes to transition state o adding a catalyst lowers the free energy of the transition state o In an uncatalyzed reaction, you must go through some sort of transition st ...
... o average energy (dashed line) of substrates (higher on graph) and products (lower on graph) o delta G starts at substrate line and goes to transition state o adding a catalyst lowers the free energy of the transition state o In an uncatalyzed reaction, you must go through some sort of transition st ...
Formation of rare earth metal complexes with Zonisamide in
... complexes with medicinal drugs plays a major role in the biological and chemical activity. Metal complexes of medicinal drugs have played a central role in the development of coordination chemistry. Metal complexes are widely used in various fields, such as biological processes, pharmaceuticals, sep ...
... complexes with medicinal drugs plays a major role in the biological and chemical activity. Metal complexes of medicinal drugs have played a central role in the development of coordination chemistry. Metal complexes are widely used in various fields, such as biological processes, pharmaceuticals, sep ...
CFCs and Alcohols
... Ethanol can also be made by reacting ethene with water. Ethene is mixed with high pressure steam in the presence of a phosphoric acid catalyst: C2H4 ...
... Ethanol can also be made by reacting ethene with water. Ethene is mixed with high pressure steam in the presence of a phosphoric acid catalyst: C2H4 ...
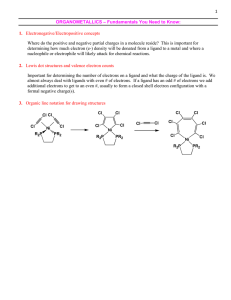
Lewis Base Ligands
... electronegative of the common halides (not counting astatine) and is the best donor group. This is some evidence that iodide is a good enough donor and has enough orbital extension to act as a 4e- and -donor in some cases. Common Misconception: Since we treat the halides as anionic halide ligands ...
... electronegative of the common halides (not counting astatine) and is the best donor group. This is some evidence that iodide is a good enough donor and has enough orbital extension to act as a 4e- and -donor in some cases. Common Misconception: Since we treat the halides as anionic halide ligands ...
11. 5-member heterocycles with 1 and heteroatoms
... Imidazole was first synthesized by Heinrich Debus in 1858, but various imidazole derivatives had been discovered as early as the 1840s. His synthesis, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole. This synthesis, while producing relatively low yields, is still used for ...
... Imidazole was first synthesized by Heinrich Debus in 1858, but various imidazole derivatives had been discovered as early as the 1840s. His synthesis, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole. This synthesis, while producing relatively low yields, is still used for ...
M - Chemistry
... to the metal. For example, the bisphosphine Ph2PCH2CH2PPh2 (dppe) is normally a chelating ligand, but there are metal complexes known where only one of the phosphine atoms is coordinated to the metal center and the other is “dangling.” The nomenclature for such a singly coordinated bisphosphine liga ...
... to the metal. For example, the bisphosphine Ph2PCH2CH2PPh2 (dppe) is normally a chelating ligand, but there are metal complexes known where only one of the phosphine atoms is coordinated to the metal center and the other is “dangling.” The nomenclature for such a singly coordinated bisphosphine liga ...
3. Organic Compounds: Alkanes and Cycloalkanes
... http://www.khanacademy.org/science/organicchemistry/v/common-and-systematic-namingiso--sec-and-tert-prefixes ...
... http://www.khanacademy.org/science/organicchemistry/v/common-and-systematic-namingiso--sec-and-tert-prefixes ...
Week of Sept. 20
... Electron Counting Step 1: Determine the oxidation state of the metal. To do this, balance the ligand charges with an equal opposite charge on the metal. This is the metal's formal oxidation state. ...
... Electron Counting Step 1: Determine the oxidation state of the metal. To do this, balance the ligand charges with an equal opposite charge on the metal. This is the metal's formal oxidation state. ...
Course No - Chemistry
... mechanistic details of the reactions involved. Reactions of alcohols including Pinacole-Pinacolone rearrangement with mechanism. Methods of formation and the oxidative cleavage reactions of diols. Phenols:- Characteristic features of Phenols.Mechanisms of Fries and Claisen rearrangements and Gatterm ...
... mechanistic details of the reactions involved. Reactions of alcohols including Pinacole-Pinacolone rearrangement with mechanism. Methods of formation and the oxidative cleavage reactions of diols. Phenols:- Characteristic features of Phenols.Mechanisms of Fries and Claisen rearrangements and Gatterm ...
Supercritical Degradation of Unsaturated Polyester Resin
... applications, water piping, building construction and automotive applications (Dholakiya, 2013). However, despite the wide applicability of FR UP composites, such materials become a crucial issue in terms of disposal routes at the end of their life cycle, exclusively because they are insoluble and i ...
... applications, water piping, building construction and automotive applications (Dholakiya, 2013). However, despite the wide applicability of FR UP composites, such materials become a crucial issue in terms of disposal routes at the end of their life cycle, exclusively because they are insoluble and i ...
Advanced Higher Chemistry Learning Outcomes
... Atomic emission spectroscopy and atomic absorption spectroscopy involve transitions between electronic energy levels in atoms. Generally, the energy differences correspond to the visible region of the electromagnetic spectrum, i.e., to the approximate wavelength range of 400-700 nm. Some application ...
... Atomic emission spectroscopy and atomic absorption spectroscopy involve transitions between electronic energy levels in atoms. Generally, the energy differences correspond to the visible region of the electromagnetic spectrum, i.e., to the approximate wavelength range of 400-700 nm. Some application ...
STUDIES OF THE LINKAGE AND BONDING OF TRIATOMICS IN
... common oxidation states) into class a acceptors, which bind most strongly with ligands containing second row elements (0, N, F etc.) as donor atoms, and class b acceptors, which form their most stable complexes with elements in higher periods of the periodic tables (P, S, Cl etc.). Exceptions to thi ...
... common oxidation states) into class a acceptors, which bind most strongly with ligands containing second row elements (0, N, F etc.) as donor atoms, and class b acceptors, which form their most stable complexes with elements in higher periods of the periodic tables (P, S, Cl etc.). Exceptions to thi ...
The Reaction Mechanism of Silicon
... ns and 6.8 µs, respectively. (3) The relative probabilities of (solvation through the ethyl moiety):(solvation through the Si-H bond) for the Mn and Re complexes are 5.3 and 3, respectively. The differences are attributed to steric effects. The larger diameter of the rhenium atom allows equivalent s ...
... ns and 6.8 µs, respectively. (3) The relative probabilities of (solvation through the ethyl moiety):(solvation through the Si-H bond) for the Mn and Re complexes are 5.3 and 3, respectively. The differences are attributed to steric effects. The larger diameter of the rhenium atom allows equivalent s ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.