chem 304 inorganic chemistry laboratory manual
... The purpose of this experiment was to synthesize a 6-coordinate cobalt(III) compound from CoCl2•6H2O. This is made difficult by the fact that Co2+ ion is more stable than Co3+ for simple salts. There are only a few salts of cobalt(III), such as CoF3, that are known. However, cobalt(III) can be made ...
... The purpose of this experiment was to synthesize a 6-coordinate cobalt(III) compound from CoCl2•6H2O. This is made difficult by the fact that Co2+ ion is more stable than Co3+ for simple salts. There are only a few salts of cobalt(III), such as CoF3, that are known. However, cobalt(III) can be made ...
d-Block Elements
... Ligands with more donor atoms make more stable complexes since more atoms have to be detached to dissociate the complex. Remember that it is the donor atoms that determine the geometry and coordinaE ...
... Ligands with more donor atoms make more stable complexes since more atoms have to be detached to dissociate the complex. Remember that it is the donor atoms that determine the geometry and coordinaE ...
Organic Nomenclature
... Combustion: react with oxygen produce carbon dioxide and water Substitution: halogen atoms replace hydrogen atoms Dehydrogenation reactions:hydrogen removed forms double bond there unsaturated hydrocarbon is the product ...
... Combustion: react with oxygen produce carbon dioxide and water Substitution: halogen atoms replace hydrogen atoms Dehydrogenation reactions:hydrogen removed forms double bond there unsaturated hydrocarbon is the product ...
Microsoft Word - Open Access Repository of Indian Theses
... components. Consequently a variety of approaches have been developed for their asymmetric and racemic synthesis. The chapter starts with the introduction and description of the various literature methods for the synthesis of various functionalized -butyrolactones. The approach towards the synthesis ...
... components. Consequently a variety of approaches have been developed for their asymmetric and racemic synthesis. The chapter starts with the introduction and description of the various literature methods for the synthesis of various functionalized -butyrolactones. The approach towards the synthesis ...
Coordination- and Redox-Noninnocent Behavior of Ambiphilic
... addition to Sb(V) centers, coordinated Sb(III) centers have also been shown to engage in secondary interactions with Lewis basic substrates.14,27 This phenomenon, which we term coordination-noninnocence, is typically manifested through the presence of low-lying Sb−X or Sb−C σ* orbitals. Drawing an a ...
... addition to Sb(V) centers, coordinated Sb(III) centers have also been shown to engage in secondary interactions with Lewis basic substrates.14,27 This phenomenon, which we term coordination-noninnocence, is typically manifested through the presence of low-lying Sb−X or Sb−C σ* orbitals. Drawing an a ...
KINETICS questions
... (a) Addition of hydrogen gas at constant temperature and volume (b) Increase in volume of the reaction vessel at constant temperature (c) Addition of catalyst. In your explanation, include a diagram of potential energy versus reaction coordinate. (d) Increase in temperature. In your explanation, inc ...
... (a) Addition of hydrogen gas at constant temperature and volume (b) Increase in volume of the reaction vessel at constant temperature (c) Addition of catalyst. In your explanation, include a diagram of potential energy versus reaction coordinate. (d) Increase in temperature. In your explanation, inc ...
Additional Information on the Synthesis of Esters
... Concentrated sulfuric acid is highly corrosive. Wear gloves and proper eye protection when using this substance. Avoid contact with skin or clothes. Use only in a fume hood. Sodium hydrogen carbonate is slightly basic, but does not pose any specific safety problems. Will decompose on the addition of ...
... Concentrated sulfuric acid is highly corrosive. Wear gloves and proper eye protection when using this substance. Avoid contact with skin or clothes. Use only in a fume hood. Sodium hydrogen carbonate is slightly basic, but does not pose any specific safety problems. Will decompose on the addition of ...
Oxygen Evolution Reaction Electrocatalysis on Transition Metal
... sustainable civilization. The hydrogen gas, an energy dense chemical, could later be used to generate electricity in a fuel cell or burned directly like natural gas.1−5 Hydrogen is also already an important feedstock for the chemical industry in processes such as petroleum refining, Fischer−Tropsch s ...
... sustainable civilization. The hydrogen gas, an energy dense chemical, could later be used to generate electricity in a fuel cell or burned directly like natural gas.1−5 Hydrogen is also already an important feedstock for the chemical industry in processes such as petroleum refining, Fischer−Tropsch s ...
Sample Exercise 24.1 Identifying the Coordination Sphere of a
... has an octahedral geometry. Like [Co(NH3)4Cl2 ]+ (Figure 24.1), it has four ligands of one type and two of another. Consequently, it possesses two isomers: one with the Cl– ligands across the metal from each other (trans-Fe(CO)4Cl2) and one with the Cl– ligands adjacent (cis-Fe(CO)4Cl2). In principl ...
... has an octahedral geometry. Like [Co(NH3)4Cl2 ]+ (Figure 24.1), it has four ligands of one type and two of another. Consequently, it possesses two isomers: one with the Cl– ligands across the metal from each other (trans-Fe(CO)4Cl2) and one with the Cl– ligands adjacent (cis-Fe(CO)4Cl2). In principl ...
Word - Chemistry and More
... d) Determine the mass percentage of each element in barium hydroxide. e) Determine the number of moles of oxygen in 49.7 grams of barium hydroxide. 13. (Chapter 10) A calorimeter containing water is used to measure the heat produced by a chemical reaction. If the water absorbs 58.5 kJ when the tempe ...
... d) Determine the mass percentage of each element in barium hydroxide. e) Determine the number of moles of oxygen in 49.7 grams of barium hydroxide. 13. (Chapter 10) A calorimeter containing water is used to measure the heat produced by a chemical reaction. If the water absorbs 58.5 kJ when the tempe ...
DOC
... (Bonding in Transition Metal Complexes) Metal complexes are usually highly colored and are often paramagnetic – such facts can be explained by a "d-orbital splitting diagram" d z2 ...
... (Bonding in Transition Metal Complexes) Metal complexes are usually highly colored and are often paramagnetic – such facts can be explained by a "d-orbital splitting diagram" d z2 ...
Coordination Compounds: Chemistry and Application
... (EDTA). The metal ions that form coordination compounds are from a group of metals known as transition metals. These metals have more than one oxidation state. This property allows the transition metals to act as Lewis acids. Coordination chemistry plays an important role in the purification of meta ...
... (EDTA). The metal ions that form coordination compounds are from a group of metals known as transition metals. These metals have more than one oxidation state. This property allows the transition metals to act as Lewis acids. Coordination chemistry plays an important role in the purification of meta ...
Chapter 20: Carboxylic Acids and Nitriles
... Reactions of Carboxylic Acids: An Overview • Carboxylic acids transfer a proton to a base to give anions, which are good nucleophiles in SN2 reactions • Like ketones, carboxylic acids undergo addition of nucleophiles to the carbonyl group • In addition, carboxylic acids undergo other reactions char ...
... Reactions of Carboxylic Acids: An Overview • Carboxylic acids transfer a proton to a base to give anions, which are good nucleophiles in SN2 reactions • Like ketones, carboxylic acids undergo addition of nucleophiles to the carbonyl group • In addition, carboxylic acids undergo other reactions char ...
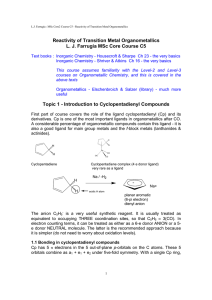
Reactivity of Transition Metal Organometallics L. J. Farrugia MSc
... The anion C5H5- is a very useful synthetic reagent. It is usually treated as equivalent to occupying THREE coordination sites, so that C5H5 ≡ 3(CO). In electron counting terms, it can be treated as either as a 6-e donor ANION or a 5e donor NEUTRAL molecule. The latter is the recommended approach bec ...
... The anion C5H5- is a very useful synthetic reagent. It is usually treated as equivalent to occupying THREE coordination sites, so that C5H5 ≡ 3(CO). In electron counting terms, it can be treated as either as a 6-e donor ANION or a 5e donor NEUTRAL molecule. The latter is the recommended approach bec ...
CHE-310 Organic Chemistry I_
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
Coordination Chemistry II: Ligand Field Theory
... σ-MOs for Tetrahedral Complexes Four-coordinate tetrahedral complexes are ubiquitous throughout the transition metals. ...
... σ-MOs for Tetrahedral Complexes Four-coordinate tetrahedral complexes are ubiquitous throughout the transition metals. ...
Slide 1
... a. Made from the fermentation of grain, fruit, or sugar b. C6H12O6 + yeast 2C2H5OH + CO2 c. The alcohol that is in beverages d. Added to automotive fuels – 10% as gasohal e. 1 pint of pure alcohol will kill most people f. caused deterioration of the liver, memory loss and is harmful to unborn babi ...
... a. Made from the fermentation of grain, fruit, or sugar b. C6H12O6 + yeast 2C2H5OH + CO2 c. The alcohol that is in beverages d. Added to automotive fuels – 10% as gasohal e. 1 pint of pure alcohol will kill most people f. caused deterioration of the liver, memory loss and is harmful to unborn babi ...
ΔG - Lemon Bay High School
... the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hydrogen and ...
... the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hydrogen and ...
Slide 1
... the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hydrogen and ...
... the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hydrogen and ...
Chem 4B First Midterm Review Sheet
... called a counter ion, which is held by electrostatic attraction. When an ion exchange resin is placed in contact with a solution containing ions, the counter ions on the resin can be replaced by an equivalent number of ions from the solution. The most common resins contain sulfonic acid groups (––SO ...
... called a counter ion, which is held by electrostatic attraction. When an ion exchange resin is placed in contact with a solution containing ions, the counter ions on the resin can be replaced by an equivalent number of ions from the solution. The most common resins contain sulfonic acid groups (––SO ...
Unit B741/02 - Modules C1, C2, C3 - Higher tier
... Ammonia, NH3, and oxygen, O2 are used to manufacture nitric acid, HNO3. Water is the other product. The reaction between ammonia and oxygen uses the following conditions: ...
... Ammonia, NH3, and oxygen, O2 are used to manufacture nitric acid, HNO3. Water is the other product. The reaction between ammonia and oxygen uses the following conditions: ...
Chapter 4
... 1. Explain how carbon’s electron configuration explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isom ...
... 1. Explain how carbon’s electron configuration explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isom ...
File - Pedersen Science
... 1. Explain how carbon’s electron configuration explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isom ...
... 1. Explain how carbon’s electron configuration explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isom ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.