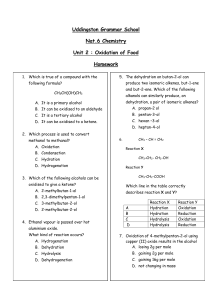
Balancing Chemical Equations
... For alcohols one hydroxyl (OH) group is substituted for one hydrogen e.g. methane becomes methyl alcohol (CH3OH) or methanol ethane becomes ethyl alcohol (C2H5OH) or ethanol ...
... For alcohols one hydroxyl (OH) group is substituted for one hydrogen e.g. methane becomes methyl alcohol (CH3OH) or methanol ethane becomes ethyl alcohol (C2H5OH) or ethanol ...
Alkanes CH4 + Cl2 → CH3Cl + HCl CH3CH3 + Cl2 → CH3CH2Cl +
... atoms for the reaction to start with, and also sterically hinders the carbon atom thus preventing the OH- from acting as a nucleophile and causing a substitution reaction ...
... atoms for the reaction to start with, and also sterically hinders the carbon atom thus preventing the OH- from acting as a nucleophile and causing a substitution reaction ...
Slide 1
... They are nonpolar molecules and consequently are not soluble in water but are soluble in typical nonpolar organic solvents like toluene or pentane. Hydrocarbons are constructed of chains or rings of carbon atoms with sufficient hydrogens to fulfill carbons need for four bonds. ...
... They are nonpolar molecules and consequently are not soluble in water but are soluble in typical nonpolar organic solvents like toluene or pentane. Hydrocarbons are constructed of chains or rings of carbon atoms with sufficient hydrogens to fulfill carbons need for four bonds. ...
Taylor`s Organic Reactions Summary Sheet
... reactants, and must be considered in designing the synthesis of specific alkyl halides. These alkyl halides can then be transformed into other organic compounds. Preparing Organic Halides: Halogenation ...
... reactants, and must be considered in designing the synthesis of specific alkyl halides. These alkyl halides can then be transformed into other organic compounds. Preparing Organic Halides: Halogenation ...
CHM 331 : General Organic Chemistry
... look from here, looks like this • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearran ...
... look from here, looks like this • ANTI addition, bromines add to opposite sides of the C=C double bond (top and bottom) • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearran ...
Aim: How can we describe Hydrocarbons?
... weak electrolytes • Have low melting points (due to weak intermolecular forces that hold them together) – The great number of carbons leads to a higher melting point. ...
... weak electrolytes • Have low melting points (due to weak intermolecular forces that hold them together) – The great number of carbons leads to a higher melting point. ...
10 Introduction to organic chemistry
... 9 In the reaction between ethene and bromine, bromine attacks the electron-rich π-bond. In ethane, all the bonds are σ-bonds, so there is no centre of high-electron density. The propagation step of the photochemical substitution reaction with ethane involves the reaction of a bromine radical with an ...
... 9 In the reaction between ethene and bromine, bromine attacks the electron-rich π-bond. In ethane, all the bonds are σ-bonds, so there is no centre of high-electron density. The propagation step of the photochemical substitution reaction with ethane involves the reaction of a bromine radical with an ...
Organic Reactions
... A) Alkenes have pi bonds in which electrons are easily accessible b/c they aren’t trapped between two nuclei as sigma bonding electrons are. ...
... A) Alkenes have pi bonds in which electrons are easily accessible b/c they aren’t trapped between two nuclei as sigma bonding electrons are. ...
Chapter 10_Organohalides
... later, but for now we will only discuss how they can be used to convert alkyl halides to alkanes • Not a very useful reaction but can eliminate halogens if necessary ...
... later, but for now we will only discuss how they can be used to convert alkyl halides to alkanes • Not a very useful reaction but can eliminate halogens if necessary ...
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
orgchem rev integ odd numbers
... bonds within a molecule or ion. Typically due to an electronegativity difference between the atoms at either end of the bond. ...
... bonds within a molecule or ion. Typically due to an electronegativity difference between the atoms at either end of the bond. ...
Carbon - Paradise High School
... • Hydrocarbons = consist only of C and H - bonds are covalent and non-polar - release lots of E when bonds are broken - don’t exist independently in living organisms, but cell’s organic molecules contain regions of H-C. ...
... • Hydrocarbons = consist only of C and H - bonds are covalent and non-polar - release lots of E when bonds are broken - don’t exist independently in living organisms, but cell’s organic molecules contain regions of H-C. ...
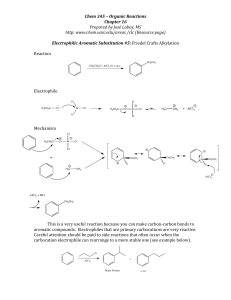
COUPLING REACTIONS IN ORGANIC SYNTHESIS
... Oxidative addition and reductive elimination are key steps in industrial catalysis. For example, both steps are featured in palladium-catalyzed cross-coupling reactions, the subject of the 2010 Nobel Prize in Chemistry. The prize was awarded to Richard Heck of the University of Delaware, Ei-Ichi Neg ...
... Oxidative addition and reductive elimination are key steps in industrial catalysis. For example, both steps are featured in palladium-catalyzed cross-coupling reactions, the subject of the 2010 Nobel Prize in Chemistry. The prize was awarded to Richard Heck of the University of Delaware, Ei-Ichi Neg ...
Naming Organic Compounds
... include both carbon atoms of the double bond. 3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. The smaller of the two numbers desig ...
... include both carbon atoms of the double bond. 3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. The smaller of the two numbers desig ...
Studying Sn1 and Sn2 reactions: Nucleophillic substitution
... The leaving group: A weak base The carbon group: unhindered by the presence of bulky groups is better for Sn2 reactions ...
... The leaving group: A weak base The carbon group: unhindered by the presence of bulky groups is better for Sn2 reactions ...
Organic Nomenclature Notes
... When naming an alkane, we start by counting the number of carbons in the longest chain in the compound. We then match the number of carbons to a prefix, and add the suffix “ane”, indicating we have an alkane. Ex) Name the following compound. CH3CH2CH2CH3 This compound has 4 carbons in it. It must ha ...
... When naming an alkane, we start by counting the number of carbons in the longest chain in the compound. We then match the number of carbons to a prefix, and add the suffix “ane”, indicating we have an alkane. Ex) Name the following compound. CH3CH2CH2CH3 This compound has 4 carbons in it. It must ha ...
Alkene/Alkyne Addition Reactions
... unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H ...
... unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.