2.10 Alcohols notes - A
... Alkenes produced in this way can be polymerised. This method therefore allows polymers to be produced without using crude oil (assuming that the original ethanol was produced by fermentation). The dehydration of alcohols is favoured by acidic conditions, as the -OH group becomes protonated by H+ ion ...
... Alkenes produced in this way can be polymerised. This method therefore allows polymers to be produced without using crude oil (assuming that the original ethanol was produced by fermentation). The dehydration of alcohols is favoured by acidic conditions, as the -OH group becomes protonated by H+ ion ...
Mill Hill County High School
... Alkenes produced in this way can be polymerised. This method therefore allows polymers to be produced without using crude oil (assuming that the original ethanol was produced by fermentation). The dehydration of alcohols is favoured by acidic conditions, as the -OH group becomes protonated by H+ ion ...
... Alkenes produced in this way can be polymerised. This method therefore allows polymers to be produced without using crude oil (assuming that the original ethanol was produced by fermentation). The dehydration of alcohols is favoured by acidic conditions, as the -OH group becomes protonated by H+ ion ...
A Diels-Alder Synthesis
... So, our Diels-Alder synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride will first involve the "cracking" of the Dicyclopentadiene into Cyclopentadiene. This product will then be immediately treated with Maleic Anhydride to carry-out our desired DielsAlder reaction. ...
... So, our Diels-Alder synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride will first involve the "cracking" of the Dicyclopentadiene into Cyclopentadiene. This product will then be immediately treated with Maleic Anhydride to carry-out our desired DielsAlder reaction. ...
Unit C
... (nitrous oxide); and analyze their contribution to climate change • draw or use models to illustrate polymers • analyze a process for producing polymers • analyze efficiencies and negative by-products related to chemical processes in organic chemistry ...
... (nitrous oxide); and analyze their contribution to climate change • draw or use models to illustrate polymers • analyze a process for producing polymers • analyze efficiencies and negative by-products related to chemical processes in organic chemistry ...
Chapter 2 Representative Carbon Compounds: Functional Groups
... Carbon’s ability to form strong covalent bonds to other carbon atoms is the single property of the carbon atom that — more than any other—accounts for the existence of a field of study called organic chemistry. Carbon’s ability to form as many as four strong bonds to other carbon atoms, and to form ...
... Carbon’s ability to form strong covalent bonds to other carbon atoms is the single property of the carbon atom that — more than any other—accounts for the existence of a field of study called organic chemistry. Carbon’s ability to form as many as four strong bonds to other carbon atoms, and to form ...
Phenols Like alcohols, phenols are starting materials for a wide
... Like alcohols, they readily form a sodium salt, but since the proton on the OH group of phenols is more acidic, the salt is formed using NaOH, not Na itself. However the salt is not formed with a weak base like Na2CO3 OH O -Na + ...
... Like alcohols, they readily form a sodium salt, but since the proton on the OH group of phenols is more acidic, the salt is formed using NaOH, not Na itself. However the salt is not formed with a weak base like Na2CO3 OH O -Na + ...
Exam 2 review sheet
... reactions of carboxylic acids: to acyl chlorides, to acid anhydrides, to esters/lactones (mechanism for Fischer esterification); to amides: direct addition of carboxylic acid plus amine is usually unproductive; relative reactivity of carboxylic acid derivatives: acid chloride > anhydride > ester > a ...
... reactions of carboxylic acids: to acyl chlorides, to acid anhydrides, to esters/lactones (mechanism for Fischer esterification); to amides: direct addition of carboxylic acid plus amine is usually unproductive; relative reactivity of carboxylic acid derivatives: acid chloride > anhydride > ester > a ...
printable - Master Organic Chemistry
... KMnO4, acid, heat cleaves C=C to give two carbonyls. Alkenyl C-H bonds oxidized to C–OH ...
... KMnO4, acid, heat cleaves C=C to give two carbonyls. Alkenyl C-H bonds oxidized to C–OH ...
Chapter 11 Lecture Notes: Alcohols, Ethers, Aldehydes, and Ketones
... bonds are broken and new ones are formed giving hairs a different shape. ...
... bonds are broken and new ones are formed giving hairs a different shape. ...
Hydrocarbons - mccormack-sch4u-2013
... Organic Compounds • Contain C bonded to other elements, commonly H, O, N, S, and halogens • Carbon – Can form many different compounds due to its hybrid orbitals – Has intermediate electonegativity, so its most likely to form molecular compounds (Recall: molecular compounds have diverse properties) ...
... Organic Compounds • Contain C bonded to other elements, commonly H, O, N, S, and halogens • Carbon – Can form many different compounds due to its hybrid orbitals – Has intermediate electonegativity, so its most likely to form molecular compounds (Recall: molecular compounds have diverse properties) ...
Amines and amides
... This reaction involves breaking the C-N bond at the carbonyl carbon. a. acid hydrolysis: forms “ammonium” ion and carboxylic acid b. alkaline hydrolysis: forms carboxylate ion and amine Condensation Polymers As we have seen –COOH (and COCl) react with amines to form 2ry amides A Condensation reactio ...
... This reaction involves breaking the C-N bond at the carbonyl carbon. a. acid hydrolysis: forms “ammonium” ion and carboxylic acid b. alkaline hydrolysis: forms carboxylate ion and amine Condensation Polymers As we have seen –COOH (and COCl) react with amines to form 2ry amides A Condensation reactio ...
Chapter 5-alcohol
... Ethers are polar compounds in which oxygen bears a partial negative charge and each carbon bonded to it bears a partial positive charge. ◦ However, only weak forces of attraction exist between ether molecules in the pure liquid. ◦ Consequently, boiling points of ethers are close to those of hydrocar ...
... Ethers are polar compounds in which oxygen bears a partial negative charge and each carbon bonded to it bears a partial positive charge. ◦ However, only weak forces of attraction exist between ether molecules in the pure liquid. ◦ Consequently, boiling points of ethers are close to those of hydrocar ...
Hydro carbons
... The simplest of the saturated cyclic hydrocarbons, or cycloalkanes, is cyclopropane, C3H6, the molecules of which are made up of three carbon atoms to each of which two hydrogen atoms are attached. Cyclopropane is somewhat more reactive than the corresponding open-chain alkane, propane, C3H8. Other ...
... The simplest of the saturated cyclic hydrocarbons, or cycloalkanes, is cyclopropane, C3H6, the molecules of which are made up of three carbon atoms to each of which two hydrogen atoms are attached. Cyclopropane is somewhat more reactive than the corresponding open-chain alkane, propane, C3H8. Other ...
Chemistry (9701/11)
... reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. To avoid the issue of disclosure of answer-related information ...
... reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included, the publisher will be pleased to make amends at the earliest possible opportunity. To avoid the issue of disclosure of answer-related information ...
Unit 3 - Organic Chemistry Straight Chain Alkanes
... Properties of Addition Polymers - addition polymers (plastics) tend to be chemically unreactive - the double bonds of the monomers are changed to the less reactive single bonds - polymer chains can ‘slide along’ each other which makes them flexible - intermolecular forces are abundant but weak so if ...
... Properties of Addition Polymers - addition polymers (plastics) tend to be chemically unreactive - the double bonds of the monomers are changed to the less reactive single bonds - polymer chains can ‘slide along’ each other which makes them flexible - intermolecular forces are abundant but weak so if ...
Asymmetric Organocatalysis
... hydrogen peroxide. In this experimentally most convenient reaction, enantiomeric excesses > 90% are readily achieved (Scheme 1.6). ...
... hydrogen peroxide. In this experimentally most convenient reaction, enantiomeric excesses > 90% are readily achieved (Scheme 1.6). ...
excess
... 4. Sodium hydride, NaH, is an ionic compound. Na H a) Write the Lewis electron-dot structure for NaH. b) If NaH is placed into water (a foolish thing to do), the hydride ion is converted to hydrogen gas (H2). The resulting solution turns red litmus paper blue and has a pH OH Na>>7.H + H2O H2 + Na Wr ...
... 4. Sodium hydride, NaH, is an ionic compound. Na H a) Write the Lewis electron-dot structure for NaH. b) If NaH is placed into water (a foolish thing to do), the hydride ion is converted to hydrogen gas (H2). The resulting solution turns red litmus paper blue and has a pH OH Na>>7.H + H2O H2 + Na Wr ...
Practice Questions - Elevate Education
... If HCl(aq) added then reaction between Cl- ions and Ag+ ions forms INSOLUBLE AgCl. The resultant drop in [Ag+] causes DROP in E0 value ...
... If HCl(aq) added then reaction between Cl- ions and Ag+ ions forms INSOLUBLE AgCl. The resultant drop in [Ag+] causes DROP in E0 value ...
Organic chemistry chapter 2
... and chemical properties Carbon combines with other atoms (e.g., H, N, O, S, halogens) to form structural units called functional groups Functional groups are important for three reason; they are 1. the units by which we divide organic compounds into classes 2. the sites of characteristic chemica ...
... and chemical properties Carbon combines with other atoms (e.g., H, N, O, S, halogens) to form structural units called functional groups Functional groups are important for three reason; they are 1. the units by which we divide organic compounds into classes 2. the sites of characteristic chemica ...
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
... saponification is irreversible because the carboxylic acid is deprotonated under the reaction conditions. ...
... saponification is irreversible because the carboxylic acid is deprotonated under the reaction conditions. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
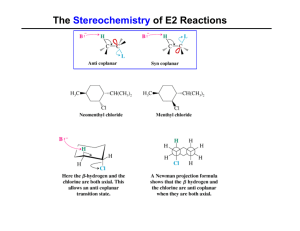
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using isotoping labeling method? 14. Write and explain the Steven’s rearrangement. 15. ...
... 11. Give an example for α, β, γ and δ-elimination reaction. 12. State and explain the Hammond postulate to the bromination of n-propane. 13. How will you determine the reaction mechanism of hydrolysis of an ester using isotoping labeling method? 14. Write and explain the Steven’s rearrangement. 15. ...
Efficient one pot synthesis of N-alkyl and N-aryl imides
... INTRODUCTION The development of simple general and efficient synthetic methods for widely used organic compounds from readily available reagents is one of the major challenges in organic synthesis. Imide derivatives are among such type of organic compound with numerous applications in biological [1] ...
... INTRODUCTION The development of simple general and efficient synthetic methods for widely used organic compounds from readily available reagents is one of the major challenges in organic synthesis. Imide derivatives are among such type of organic compound with numerous applications in biological [1] ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.