* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hydrocarbons - mccormack-sch4u-2013
Survey
Document related concepts
Transcript
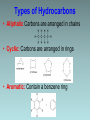
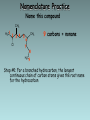
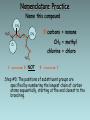
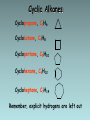
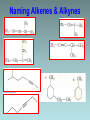
Organic Chemistry Organic chemistry is the study of carbon containing compounds derived from living organisms. Organic Compounds • Contain C bonded to other elements, commonly H, O, N, S, and halogens • Carbon – Can form many different compounds due to its hybrid orbitals – Has intermediate electonegativity, so its most likely to form molecular compounds (Recall: molecular compounds have diverse properties) – Can make single, double, and triple bonds – Can form isomers (same molecular formula but different arrangement of atoms) Isomers • Structural Isomers have the same molecular formula but the atoms are bonded together in a different sequence • Stereoisomers are molecules with the same molecular formula, a same sequence of atoms but they have different 3D orientations Stereoisomers • Diastereomers form around a double bond and each carbon atom involved in the bond must have different types of atoms bonded to it • Enantiomers are mirror images of each other Types of Hydrocarbons • Saturated: Contain the maximum number of hydrogens, single bonds between all carbons • Unsaturated: Contain 1+ double or triple bonds Types of Hydrocarbons • Aliphatic Carbons are arranged in chains • Cyclic: Carbons are arranged in rings • Aromatic: Contain a benzene ring Types of Hydrocarbons Name Alkane Alkene Alkyne Definition Hydrocarbon with only single bonds between carbon atoms. Hydrocarbon with at least one double bond. Hydrocarbon with at least one triple bond. General Formula CnH2n+2 CnH2n CnH2n-2 • Homologous series • This is a series of compounds which all contain the same functional group, and have similar chemical properties. • ALKANES ALKENES ALCOHOLS • CH4 CH2 =CH2 CH3OH • CH3-CH3 CH2 =CH –CH3 CH3CH2OH • Each has a general formula: • ALKANES: CnH2n+2 • The members of the series differ by the number of CH2 units. • CH3-CH3, CH3-CH2-CH3, CH3-CH2-CH2-CH3 • Graduation in physical properties: eg: boiling points. • CH4 (GAS), C8H18 (LIQUID), C30H62 (SOLID) Rules for Naming Alkanes (Nomenclature) For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon H3C1 2 H3C 3 4 CH3 4 carbon chain = butane Naming Alkanes Based off the number of C atoms in the longest chain 1. Count the number of C’s in the longest chain 2. Determine the appropriate root 3. Add the suffix “ane” Hydrocarbon Root Names # of Carbons 1 2 3 4 5 6 7 8 9 10 Root Name methethpropbutpenthexheptoctnondec- Examples • Butane • Heptane Naming Branched Alkanes Based off the number of C atoms in the longest chain 1. Count the number of C’s in the longest chain 2. Determine the appropriate root 3. Use the numbered C’s to give the branches a position number add “yl” suffix 4. Add the suffix “ane” Naming Branched Alkanes Important Rules: 1. Start numbering from the end that will give you the lowest number of branches 2. If there is more than one type of branch, name the branches in alphabetical order 3. If there is more than two of the same type of branch, give the branch a position number and prefixes “di”, “tri” “tetra” etc. 4. Put commas between numbers and hyphens between numbers and letters Rules for Naming Alkanes (Nomenclature) When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. —CH3 Methyl —CH2CH3 Ethyl —CH2CH2CH3 Propyl —CH2CH2CH2CH3 Butyl H3C H3C Methyl CH3 Rules for Naming Alkanes (Nomenclature) The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. H3C1 2 3 H3C Methyl 4 CH3 Rules for Naming Alkanes (Nomenclature) The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. H3C1 2 3 H3C Methyl 4 CH3 Name: 2-methylbutane Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 9 carbons = nonane 8 H3C9 Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 8 9 carbons = nonane CH3 = methyl chlorine = chloro H3C9 Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 6 7 9 carbons = nonane CH3 CH3 = methyl chlorine = chloro 8 H3C9 1 9 NOT 9 1 Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 7 9 carbons = nonane CH3 = methyl 8 chlorine = chloro H3C9 2-chloro-3,6-dimethylnonane Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. Cyclic Alkanes Cyclopropane, C3H6 Cyclobutane, C4H8 Cyclopentane, C5H10 Cyclohexane, C6H12 Cycloheptane, C7H14 Remember, explicit hydrogens are left out Practice • P. 11-16 #1, 2 Structural Shorthand Explicit hydrogens (those required to complete carbon’s valence) are usually left off of drawings of hydrocarbons H CH3 H H H H3C H C1 C2 C3 C4 H H H H H C1 C2 C3 C4 Line intersections represent carbon atoms Different Methods of Displaying Compounds • Molecular Formula • Expanded Molecular Formula • Condensed Structural Formula • Structural Formula • Line Formula Naming Alkenes & Alkynes 1. Count the number of C’s in the longest chain containing the double/triple bond. • • This is the parent chain, determine the root Number the parent chain so that the double/triple bond has the lowest possible position number 2. Identify the position numbers of branches • Same rules as before 3. Write the branches in alphabetical order 4. Write the root, including a prefix that identifies the position of the double/triple bond • Add the prefix “cyclo” if its cyclic 5. Add the suffix “ene” or “yne” Naming Alkenes & Alkynes Naming Aromatics 1. Same rules 2. If benzene is the parent chain “benzene” suffix 3. If benzene is a branch group “phenyl” Practice • • • • P. 16-22 #3-7, 8abc Naming Alkenes/Alkynes Worksheet Isomer Challenge Worksheet Naming Hydrocarbons Worksheet