Chemdraw B&W - Pennsylvania State University
... base, {C5H11NO2}, produced artificially, and also occurring naturally in beet-root molasses and its residues. The listed pronunciation indicates it has the exact same emphasis as “cocaine”. • Cocaine \Co"ca*ine\, n. (Chem.) A powerful alkaloid, {C17H21NO4}, obtained from the leaves of coca • So – if ...
... base, {C5H11NO2}, produced artificially, and also occurring naturally in beet-root molasses and its residues. The listed pronunciation indicates it has the exact same emphasis as “cocaine”. • Cocaine \Co"ca*ine\, n. (Chem.) A powerful alkaloid, {C17H21NO4}, obtained from the leaves of coca • So – if ...
Unsaturated Hydrocarbons I : Alkenes
... of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. In cycloalkenes the double bond carbons are assigned ring locations C1 and C2. Which of the two is C1 may be determined by the nearest substituent rule. 5. Substituent groups containing double bonds ar ...
... of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. In cycloalkenes the double bond carbons are assigned ring locations C1 and C2. Which of the two is C1 may be determined by the nearest substituent rule. 5. Substituent groups containing double bonds ar ...
Chapter 20. Aldehydes and Ketones
... compound (C6H5)2CHOH by adding C6H5MgBr to benzaldehyde. To insure that the chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline produc ...
... compound (C6H5)2CHOH by adding C6H5MgBr to benzaldehyde. To insure that the chemical yield would be high, our dedicated student prepared one mole of the Grignard reagent, added two moles of benzaldehyde, and, after working up the reaction, was delighted to obtain a good yield of a crystalline produc ...
Efficient and catalyst-free condensation of acid chlorides and
... An efficient and catalyst-free procedure for the condensation of acyl chlorides and alcohols using continuous flow was developed. Different esters could be obtained with excellent conversions starting from the corresponding acyl chlorides and alcohols in very short reaction times (5-6.7 min). The re ...
... An efficient and catalyst-free procedure for the condensation of acyl chlorides and alcohols using continuous flow was developed. Different esters could be obtained with excellent conversions starting from the corresponding acyl chlorides and alcohols in very short reaction times (5-6.7 min). The re ...
Part 1
... Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity Organic halides: a hydrogen is replaced by a halogen (fluoro-, chloro-, bromo-, iodo-) 2-iodobutane 2,4-dibromo-1-hexene 1-bromo-2-chlorobenzene ...
... Functional groups: special groups of atoms attached to a hydrocarbon skeleton; the most common sites of chemical reactivity Organic halides: a hydrogen is replaced by a halogen (fluoro-, chloro-, bromo-, iodo-) 2-iodobutane 2,4-dibromo-1-hexene 1-bromo-2-chlorobenzene ...
Alcohols
... This reaction does not always give good yields of RX. 1° and 2° alcohols react slowly with HCl, even with ZnCl2 added. Heating an alcohol with HCl or HBr can give the elimination product, an alkene. Rearrangements can occur with SN1 (this is not necessarily bad). HI does not give good yiel ...
... This reaction does not always give good yields of RX. 1° and 2° alcohols react slowly with HCl, even with ZnCl2 added. Heating an alcohol with HCl or HBr can give the elimination product, an alkene. Rearrangements can occur with SN1 (this is not necessarily bad). HI does not give good yiel ...
Chabot College
... Continuation of Chemistry 12A with an introduction to the chemistry of dienes, aromatics, amines, carbanions, carboxylic acids, carboxylic acid derivatives, aldehydes, ketones and biochemical topics focusing on structure, synthesis, and mechanisms of reaction. Laboratory work in basic techniques, sy ...
... Continuation of Chemistry 12A with an introduction to the chemistry of dienes, aromatics, amines, carbanions, carboxylic acids, carboxylic acid derivatives, aldehydes, ketones and biochemical topics focusing on structure, synthesis, and mechanisms of reaction. Laboratory work in basic techniques, sy ...
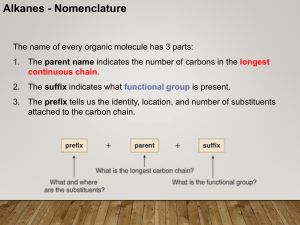
CHEM 210 Nomenclature Lecture 1
... Alkanes - Nomenclature Nomenclature—Common Names Some organic compounds are identified using common names that do not follow the IUPAC system of nomenclature. Many of these names were given long ago before the IUPAC system was adopted, and are still widely used. Additionally, some names are descrip ...
... Alkanes - Nomenclature Nomenclature—Common Names Some organic compounds are identified using common names that do not follow the IUPAC system of nomenclature. Many of these names were given long ago before the IUPAC system was adopted, and are still widely used. Additionally, some names are descrip ...
IOSR Journal of Applied Chemistry (IOSR-JAC) ISSN: 2278-5736.
... Protection could be regarded as a special instance of a combine chemo and region selectivity since it embrace aspects of both. It is implicated when a reaction selectivity at one functional group is needed in the presence of other functional group. When the principles of chemo selectivity are not ap ...
... Protection could be regarded as a special instance of a combine chemo and region selectivity since it embrace aspects of both. It is implicated when a reaction selectivity at one functional group is needed in the presence of other functional group. When the principles of chemo selectivity are not ap ...
3: Haloalkanes, Alcohols, Ethers, and Amines
... The relative magnitudes of these electronegativity differences reflect the relative magnitudes of the polarity of each bond. The negative (-) electronegativity difference for a C-H bond suggests that C is (δ-) while H is (δ+), however the magnitude of the electronegativity difference is so small tha ...
... The relative magnitudes of these electronegativity differences reflect the relative magnitudes of the polarity of each bond. The negative (-) electronegativity difference for a C-H bond suggests that C is (δ-) while H is (δ+), however the magnitude of the electronegativity difference is so small tha ...
Chem 216 H W13 Notes - Dr. Masato Koreeda Thin
... (3) Iodine jar: C=C bond-containing compounds can be detected by exposing the dried, developed TLC to the iodidne vapor. ...
... (3) Iodine jar: C=C bond-containing compounds can be detected by exposing the dried, developed TLC to the iodidne vapor. ...
Pre-lab Questions - HCC Learning Web
... Melting (Fusion), Boiling points and Density- Straight-chain alcohols that have up to 12 carbons have boiling points that are considerably higher than the related alkanes, Alcohols with two or more –OH groups have higher boiling points. Because the structural similarities between alcohols and water, ...
... Melting (Fusion), Boiling points and Density- Straight-chain alcohols that have up to 12 carbons have boiling points that are considerably higher than the related alkanes, Alcohols with two or more –OH groups have higher boiling points. Because the structural similarities between alcohols and water, ...
Lecture 17-edited
... Hydrogen is the most abundant chemical element in the universe with atomic number 1 and symbol H. It has three isotopes hydrogen 1H, deuterium 2D and tritium 3T and the 1H is the most abundant (99.98%). The hydrogen 1H and deuterium 2D are stable isotopes whereas the tritium ...
... Hydrogen is the most abundant chemical element in the universe with atomic number 1 and symbol H. It has three isotopes hydrogen 1H, deuterium 2D and tritium 3T and the 1H is the most abundant (99.98%). The hydrogen 1H and deuterium 2D are stable isotopes whereas the tritium ...
EXPERIMENT 5: Oxidation of Alcohols: Solid
... relationship has led to the development of a convenient qualitative test for distinguishing primary and secondary alcohols (and aldehydes) from tertiary alcohols (and ketones). The qualitative test involves the addition of a solution of CrO3 in sulfuric acid (Jones' Reagent) to a solution of the com ...
... relationship has led to the development of a convenient qualitative test for distinguishing primary and secondary alcohols (and aldehydes) from tertiary alcohols (and ketones). The qualitative test involves the addition of a solution of CrO3 in sulfuric acid (Jones' Reagent) to a solution of the com ...
www.xtremepapers.net
... The preparations of benzamide (see Unit 9B), and N-phenylethanamide produce readily recrystallisable amides, and are good examples of this nucleophilic substitution (condensation) reaction. The above two amides can be hydrolysed (although with either OH- or H+ it takes several hours), and a good sam ...
... The preparations of benzamide (see Unit 9B), and N-phenylethanamide produce readily recrystallisable amides, and are good examples of this nucleophilic substitution (condensation) reaction. The above two amides can be hydrolysed (although with either OH- or H+ it takes several hours), and a good sam ...
Chem 350 Jasperse Ch. 6 Summary of Reaction Types, Ch. 4
... Often an atom gives up an old bond and replaces it with a new bond. This is “substitution”. In this case, there will be an incoming arrow pointing directly at the atom (to illustrate formation of the new bond), and an outgoing arrow emanating from the old bond that breaks ...
... Often an atom gives up an old bond and replaces it with a new bond. This is “substitution”. In this case, there will be an incoming arrow pointing directly at the atom (to illustrate formation of the new bond), and an outgoing arrow emanating from the old bond that breaks ...
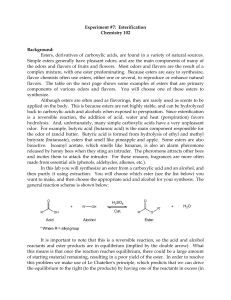
ESTERIFICATION
... bioactive. Isoamyl acetate, which smells like bananas, is also an alarm pheromone released by honey bees when they sting an intruder. The pheromone attracts other bees and incites them to attack the intruder. For these reasons, fragrances are more often made from essential oils (phenols, aldehydes, ...
... bioactive. Isoamyl acetate, which smells like bananas, is also an alarm pheromone released by honey bees when they sting an intruder. The pheromone attracts other bees and incites them to attack the intruder. For these reasons, fragrances are more often made from essential oils (phenols, aldehydes, ...
Diels-Alder Reaction
... http://www.chem.ufl.edu/~barbaro/2211L/diels-alder/da-proc.html The Diels-Alder reaction is probably the most familiar example of a reaction type known as a cycloaddition reaction, in which the conjugated p-systems of two reactants join to generate a new ring. The reactants in the Diels-Alder reacti ...
... http://www.chem.ufl.edu/~barbaro/2211L/diels-alder/da-proc.html The Diels-Alder reaction is probably the most familiar example of a reaction type known as a cycloaddition reaction, in which the conjugated p-systems of two reactants join to generate a new ring. The reactants in the Diels-Alder reacti ...
Chapter-1 ALCOHOLS
... carbonyl compounds. The choice of carbonyl type (ketone, aldehyde, ester, etc) and the type of reaction (Grignard addition or Reduction), will determine the product(s) you will get. There are primarily two types of reactions used to create alcohols from carbonyls: Grignard Addition reactions and Red ...
... carbonyl compounds. The choice of carbonyl type (ketone, aldehyde, ester, etc) and the type of reaction (Grignard addition or Reduction), will determine the product(s) you will get. There are primarily two types of reactions used to create alcohols from carbonyls: Grignard Addition reactions and Red ...
chapter19
... Aldehyde hydrate is oxidized to a carboxylic acid by usual reagents for alcohols ...
... Aldehyde hydrate is oxidized to a carboxylic acid by usual reagents for alcohols ...
Lab 7_Esterification
... bioactive. Isoamyl acetate, which smells like bananas, is also an alarm pheromone released by honey bees when they sting an intruder. The pheromone attracts other bees ...
... bioactive. Isoamyl acetate, which smells like bananas, is also an alarm pheromone released by honey bees when they sting an intruder. The pheromone attracts other bees ...
part 1
... Aldehydes form hydrates in water (an equilibrium) l An aldehyde hydrate is a diol and can react by the same ...
... Aldehydes form hydrates in water (an equilibrium) l An aldehyde hydrate is a diol and can react by the same ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.