102 Lecture Ch14b
... • Primary alcohols are initially oxidized to aldehydes, but aldehydes are easily oxidized to carboxylic acids • In order to stop the reaction at the aldehyde a very mild reducing agent must be used • The most common reagent is PCC (pyridinium chlorochromate) • Other oxidizing agents (like CrO3/H3O+) ...
... • Primary alcohols are initially oxidized to aldehydes, but aldehydes are easily oxidized to carboxylic acids • In order to stop the reaction at the aldehyde a very mild reducing agent must be used • The most common reagent is PCC (pyridinium chlorochromate) • Other oxidizing agents (like CrO3/H3O+) ...
Oxidation and Reduction Reactions
... group (the carbonyl) and left others alone (e.g. alkenes). If we want to reduce an alkene or alkyne, we need to use a different kind of hydrogen source – one which is not chemoselective but will add hydrogen across any bond. We want our hydrogen source to be, quite literally, hydrogen (H2). Proble ...
... group (the carbonyl) and left others alone (e.g. alkenes). If we want to reduce an alkene or alkyne, we need to use a different kind of hydrogen source – one which is not chemoselective but will add hydrogen across any bond. We want our hydrogen source to be, quite literally, hydrogen (H2). Proble ...
inorganic chemistry
... extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface. The high reactivity of fluorine means that once it does react with something, it bonds with it so strongly that the resulting molecule is very inert and non-reactive to anything else. F ...
... extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface. The high reactivity of fluorine means that once it does react with something, it bonds with it so strongly that the resulting molecule is very inert and non-reactive to anything else. F ...
Programme
... The discovery and mechanistic investigation of a new class of photochemical reactions of benzophenones and related compounds is documented in this Thesis. Their photobehaviour in aqueous solvent media varied dramatically from their well-known behaviour in organic solvents and suggests unique and unp ...
... The discovery and mechanistic investigation of a new class of photochemical reactions of benzophenones and related compounds is documented in this Thesis. Their photobehaviour in aqueous solvent media varied dramatically from their well-known behaviour in organic solvents and suggests unique and unp ...
Chem 150 Unit 4 - Chemical Properties I Chemical Reactions
... * The elements in Group IIA form compounds (such as Mg3N2 and CaCO3) in which the metal atom is in the +2 oxidation state. * Oxygen usually has an oxidation number of -2. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O2, O3, H2O2, and the O22- ion. * The nonmetals ...
... * The elements in Group IIA form compounds (such as Mg3N2 and CaCO3) in which the metal atom is in the +2 oxidation state. * Oxygen usually has an oxidation number of -2. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O2, O3, H2O2, and the O22- ion. * The nonmetals ...
Amines By
... Primary: the “R” is only bonded in one place in this type of bond Secondary: The “R” is bonded in two places in this bond Tertiary: The “R” is bonded in three places in this bond. ...
... Primary: the “R” is only bonded in one place in this type of bond Secondary: The “R” is bonded in two places in this bond Tertiary: The “R” is bonded in three places in this bond. ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Summary (comparison to other methods) ...
... •Summary (comparison to other methods) ...
Solvent free permanganate oxidations
... of sulfones (Scheme 3) which are important intermediates in the synthesis of many organic compounds.15 The observation that benzyl phenyl sulfide is oxidized to the corresponding sulfone indicates that the reaction proceeds by way of an oxygen transfer mechanism. If the reaction involved electron tr ...
... of sulfones (Scheme 3) which are important intermediates in the synthesis of many organic compounds.15 The observation that benzyl phenyl sulfide is oxidized to the corresponding sulfone indicates that the reaction proceeds by way of an oxygen transfer mechanism. If the reaction involved electron tr ...
organic chemistry reaction scheme
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
or H - No Brain Too Small
... polymerisation (limited to addition polymerisation @ level 2) o unsaturated monomers joined to make a polymer (saturated) o it is an addition reaction as the monomers add (and no other product made) ...
... polymerisation (limited to addition polymerisation @ level 2) o unsaturated monomers joined to make a polymer (saturated) o it is an addition reaction as the monomers add (and no other product made) ...
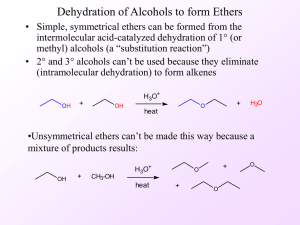
ETHERS
... ROH + R'OH )))))))))> ROR + ROR' + R'OR' + H2O However, if one alcohol is t-BuOH, t-Bu-O-R will be the major product owing to rapid formation of t-Bu+ carbocation and inability to form t-Bu-O-t-Bu. ...
... ROH + R'OH )))))))))> ROR + ROR' + R'OR' + H2O However, if one alcohol is t-BuOH, t-Bu-O-R will be the major product owing to rapid formation of t-Bu+ carbocation and inability to form t-Bu-O-t-Bu. ...
Lesson 1 Theme: Classification and nomenclature of organic
... This type of nomenclature is the most convenient one for such classes as ethers, sulfides, amines, and halogen compounds, especially for the compounds with simple radicals. 1.2.2. General Principles of Forming a Systematic Name The formation of a name for a chemical compound usually involves the fol ...
... This type of nomenclature is the most convenient one for such classes as ethers, sulfides, amines, and halogen compounds, especially for the compounds with simple radicals. 1.2.2. General Principles of Forming a Systematic Name The formation of a name for a chemical compound usually involves the fol ...
EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
Dehydration of Alcohols - Dehydration of Cyclohexanol
... undergo a 1,2-elimination reactions to generate an alkene and water. Also known as dehydration since it involves the removal of a molecule of water. Alcohol relative reactivity order: 3o > 2 o > 1 o e) Regioselectivity: major product is usually the more highly substituted alkene (alkene stability) Z ...
... undergo a 1,2-elimination reactions to generate an alkene and water. Also known as dehydration since it involves the removal of a molecule of water. Alcohol relative reactivity order: 3o > 2 o > 1 o e) Regioselectivity: major product is usually the more highly substituted alkene (alkene stability) Z ...
Acid-Catalyzed Dehydration of Alcohols
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
... as a layer on the surface of the acid-alcohol mixture (the density of the alkene is less than one); the gas can then be collected over water or the liquid layer can be removed by simple distillation to give the final alkene product. In alcohols where there are more than two kinks of -hydrogens, the ...
Study Materials
... 22) Why are alkene and akynes more reactive compared to alkanes 23) Explain addition of hydrogen to ethene 24) Write the name and structural formula of product formed by addition of bromine to ethene 25) What does the root word of IUPAC name of organic compound indicate? ...
... 22) Why are alkene and akynes more reactive compared to alkanes 23) Explain addition of hydrogen to ethene 24) Write the name and structural formula of product formed by addition of bromine to ethene 25) What does the root word of IUPAC name of organic compound indicate? ...
NaBH4 Reduction of Vanillin
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
11/29 Lecture
... Are there differences between cis- and transisomers? Large differences in melting points for example between natural plant fatty acids (almost always cis-) and trans- fatty acids formed as a bi-product of hydrogenation (partial conversion from alkenes to alkanes) Trans- forms have very small kink vs ...
... Are there differences between cis- and transisomers? Large differences in melting points for example between natural plant fatty acids (almost always cis-) and trans- fatty acids formed as a bi-product of hydrogenation (partial conversion from alkenes to alkanes) Trans- forms have very small kink vs ...
Identification of Alcohols
... obtain the iodoform precipitate. It is important to proceed through all these steps so that only at the final step you can say that the test is negative. Both ethanol and sec-butanol give positive iodoform test and they can be differentiated only by testing their solubility in water; secbutanol ...
... obtain the iodoform precipitate. It is important to proceed through all these steps so that only at the final step you can say that the test is negative. Both ethanol and sec-butanol give positive iodoform test and they can be differentiated only by testing their solubility in water; secbutanol ...
CH - cloudfront.net
... Alkyl Group This group does not exist independently but occurs bonded to another atom or molecule. H H ...
... Alkyl Group This group does not exist independently but occurs bonded to another atom or molecule. H H ...
File - Riske Science
... • Hydrocarbons are organic compounds that are made up of only carbon and ...
... • Hydrocarbons are organic compounds that are made up of only carbon and ...
acidic site
... It is, however, entirely possible for a molecule to be both an acid and an electrophile – or both a base and a nucleophile. It is even possible for the same molecule to be an acid, a base, a nucleophile and an electrophile all at the same time. In that case, how it reacts will depend on the other sp ...
... It is, however, entirely possible for a molecule to be both an acid and an electrophile – or both a base and a nucleophile. It is even possible for the same molecule to be an acid, a base, a nucleophile and an electrophile all at the same time. In that case, how it reacts will depend on the other sp ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.























