* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHAPTER 8 - REACTION EXAMPLES (Based on the 6th edition of
Survey
Document related concepts
Transcript
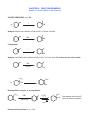
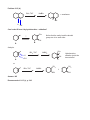
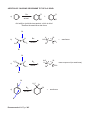
CHAPTER 8 - REACTION EXAMPLES (Based on the 6th edition of the textbook) SOLVED PROBLEM 8-1, p. 329 ? a) Br Analysis: Markovnikov addition of HBr to the C=C bond. Use HBr: HBr Br Complement: ? Br Analysis: Anti-Markovnikov addition of HBr to the C=C bond. Use HBr in the presence of peroxides HBr ROOR CH 3 OH b) ? Br CH 3 Br Retrosynthetic analysis, or retrosynthesis: CH 3 Br HBr H 2SO 4 peroxides H 3O Recommended problem: 8-4, p. 330 CH 3 OH Concentrated acid favors E1, dilute acid favors hydration OXYMERCURATION - DEMERCURATION, ALSO CALLED OXYMERCURATION - REDUCTION Hg(AcO)2 NaBH 4 a) HO Markovnikov alcohol H 2O OH Hg(AcO)2 b) NaBH 4 Markovnikov alcohol without rearrangement H 2O Contrast with addition of water in the presence of strong acid: H 3O OH H 2O 3o cation 2o cation Rearranged alcohol OH Hg(AcO)2 c) NaBH 4 CH 3 H 2O HYDROBORATION - OXIDATION SEQUENCE. An effective way to make Anti-Markovnikov alcohols. Water adds to the double bond with syn-stereochemistry. B2H 6 a) H 2O 2 Anti-Markovnikov alcohol or: BH 3 / THF OH SOLVED PROBLEM 8-3, p. 339 BH 3 / THF H 2O 2 OH CH 3 H H OH CH 3 H OH H CH 3 CH 3 + or: OH OH trans isomer CHIRAL! Enantiomer Problem 8-15 (b) BH 3, THF NaBH 4 + enantiomer H H OH OH Can I make B from A by hydroboration - oxidation? CH 3 ? Notice that the methyl and the alcohol group are cis to each other. OH B A Analysis: BH 3, THF CH 3 H NaBH 4 H A OH OH Hydroborationoxidation yields the trans alcohol. H or: BH 3, THF NaBH 4 + OH Answer: NO Recommended: 8-15 (c), p. 342 CH 3 CH 3 OH ADDITION OF CHLORINE OR BROMINE TO THE C=C BOND. CL2 Cl Cl a) + solvent Cl Cl Anti addition yields the trans product, which is chiral. Therefore the enantiomer also forms. Br H b) H C C CH 3 H 3C H H 3C Br2 solvent S C S Br + C Br H enantiomer CH 3 Br Br c) H 3C H C C CH 3 H H 3C H Br2 solvent R C Br S Br meso compound (no enantiomer) C H CH 3 Br Br H 3C Br2 d) solvent Br Recommended: 8-17, p. 345 Br Br + enantiomer Variation with water: OH H 3C OH Br Br2 + enantiomer H 2O Br Recommended: 8-5 and 8-6, p. 347 CATALYTIC HYDROGENATION - Transformation of alkenes into alkanes (syn addition of hydrogen). H2 Pt, Pd, or Ni a) CH 3 H2 b) Pd CH 3 H2 c) Pd Recommended: 8-23, p. 350 SYN HYDROXYLATION - Syn addition of OH / OH to the C=C bond. KMnO 4 cold, dilute CH 3 OH OH CH 3 MILD OXIDATION OsO 4 H 2O 2 CH 3 OH OH CH 3 meso form, no enantiomer ANTI HYDROXYLATION SEQUENCE- Anti addition of OH / OH to the C=C bond. MCPCBA a) OH H 3O O + metachloro perbenzoic acid enantiomer OH epoxide (3-membered cyclic ether) b) H H C MCPBA C H 3C CH 3 O H H H 3C CH 3 H 3O HO H C H 3C C CH 3 MCPBA H O H CH 3 H 3C d) H 3O H HO H CH 3 R S H H 3C OH meso CH 3 CH 3 O OH chiral trans epoxide MCPBA CH 3 H H H 3C cis epoxide c) R R H 3O OH OH Recommended for syn and anti hydroxilation: 8-34 (all), p. 359. + enantiomer OXIDATIVE CLEAVAGE: Strong oxidation with potassium permanganate. In this reaction each of the sp2 carbons involved in the pi bond gets oxidized to its maximum possible oxidation state. Refer to notes set # 20 (Oxidation and Reduction in Organic Chemistry) to find out what these states are. O KMnO 4 a) hot, concentrated CO 2 O KMnO 4 b) + OH D HO OH + O O O c) KMnO 4 OH D OH OH or: HO O O O O d) KMnO 4 CH 3 D OH O e) O KMnO 4 D 2 OH or: H 3C O OXIDATIVE CLEAVAGE: Ozonolysis In this reaction each of the sp2 carbons involved in the pi bond gets oxidized either to aldehyde or ketone, depending on whether it ends up at the end of a carbon chain or in the middle after the pi bond cleaves. If the oxidized carbon ends up at the end of a carbon chain it becomes an aldehyde, otherwise it becomes a ketone. O O 1) O 3 a) 2) Me2S Me = methyl + H H H formaldehyde acetaldehyde O H 1) O 3 b) + H 2) Me2S O propionaldehyde acetaldehyde O c) 1) O 3 CHO CHO 2) Me2S or: H H O O O d) e) 1) O 3 CH 3 2) Me2S CHO 1) O 3 O H or: H 3C O 2 2) Me2S Recommended strong oxidation (oxidative cleavage) with KMnO4 and with ozone: 8-7, 8-36, and 8-37, p. 362-363. Recommended problems from the end of the chapter: 47 (all), 49 ( a-f ), 58 (all), 63.