File
... The effervescence caused by production of CO2 with carboxylic acids with solid Na2CO3 or aqueous NaHCO3 can be used as a functional group test for carboxylic acids ...
... The effervescence caused by production of CO2 with carboxylic acids with solid Na2CO3 or aqueous NaHCO3 can be used as a functional group test for carboxylic acids ...
Alcohols, Phenols , Phenols and Ethers Alcohols
... hence simpler. Therefore, let us first learn how are alcohols, phenols and ethers classified? Alcohols and phenols may be classified as mono–, di–, tri- or polyhydric compounds depending on whether they contain one, two, three or many hydroxyl groups respectively in their structures as given below: ...
... hence simpler. Therefore, let us first learn how are alcohols, phenols and ethers classified? Alcohols and phenols may be classified as mono–, di–, tri- or polyhydric compounds depending on whether they contain one, two, three or many hydroxyl groups respectively in their structures as given below: ...
Unit-8-Alcohols-Aldehydes-Ketones
... organic molecules that play important roles in biochemistry; looking both at their physical and chemical properties. The Group VIA elements, oxygen and sulfur, typically form two covalent bonds to attain a filled valence shell. The families that include oxygen and sulfur with two single bonds includ ...
... organic molecules that play important roles in biochemistry; looking both at their physical and chemical properties. The Group VIA elements, oxygen and sulfur, typically form two covalent bonds to attain a filled valence shell. The families that include oxygen and sulfur with two single bonds includ ...
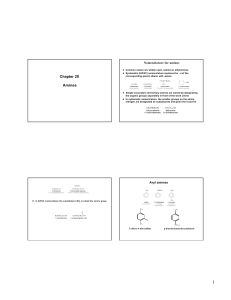
amine
... AMINES Nitrogen containing compound Nitrogen is a group 5A element with 5 valence electrons Nitrogen has 3 bonds and 1 lone pair Ammonia derivatives where at least one H is replaced by an alkyl, cycloalkyl, or aryl group ...
... AMINES Nitrogen containing compound Nitrogen is a group 5A element with 5 valence electrons Nitrogen has 3 bonds and 1 lone pair Ammonia derivatives where at least one H is replaced by an alkyl, cycloalkyl, or aryl group ...
New synthetic methodologies based on active transition metals*
... In the last five years, there has been increasing interest in the transition-metal-catalyzed α-alkylation of ketones with primary alcohols, as an alternative strategy to the standard α-alkylation of ketone enolates with alkyl halides, which can improve the regioselectivity of the process at the time ...
... In the last five years, there has been increasing interest in the transition-metal-catalyzed α-alkylation of ketones with primary alcohols, as an alternative strategy to the standard α-alkylation of ketone enolates with alkyl halides, which can improve the regioselectivity of the process at the time ...
98 pts
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
Production of NMOCs and Trace Organics During the
... Ultimate NMOC yields vary substantially among MSW components Lab-scale NMOC and HAP yields are considerably lower than regulatory estimates NMOC production is characterized by an initial “burst”, followed by much more gradual release ...
... Ultimate NMOC yields vary substantially among MSW components Lab-scale NMOC and HAP yields are considerably lower than regulatory estimates NMOC production is characterized by an initial “burst”, followed by much more gradual release ...
A Biocatalytic Henry Reaction-The Hydroxynitrile Lyase from Hevea
... HNLs from different sources have been identified, and the HNL-catalyzed synthesis of a large number of cyanohydrins with R and S configuration with excellent stereoselectivity has been demonstrated.[2] In trying to expand the synthetic applicability of the HNL methodology we considered replacing HCN ...
... HNLs from different sources have been identified, and the HNL-catalyzed synthesis of a large number of cyanohydrins with R and S configuration with excellent stereoselectivity has been demonstrated.[2] In trying to expand the synthetic applicability of the HNL methodology we considered replacing HCN ...
CfE Advanced Higher Chemistry Unit 2: Organic
... The energy required to promote the electron would be more than offset by the formation of two extra covalent bonds. However, whereas the others would involve 2p orbitals. Spectroscopic measurements show that all four bonds in methane are identical. Let's look at an alkane, ethane for example. Each c ...
... The energy required to promote the electron would be more than offset by the formation of two extra covalent bonds. However, whereas the others would involve 2p orbitals. Spectroscopic measurements show that all four bonds in methane are identical. Let's look at an alkane, ethane for example. Each c ...
Chapter 3
... • Final “e” is replaced with “amine” • Number the carbon to which nitrogen is bonded • Number any substituents on the alkyl chain • Use italicized N- for each additional substituent(s) on the nitrogen Chapter 3 ...
... • Final “e” is replaced with “amine” • Number the carbon to which nitrogen is bonded • Number any substituents on the alkyl chain • Use italicized N- for each additional substituent(s) on the nitrogen Chapter 3 ...
Petrochemicals - MullisChemistry
... Unsaturated hydrocarbons – Double and triple bonds between carbons – Not every carbon has each of its 4 electrons bonded to 4 different atoms – More chemically reactive than saturated compounds, or alkanes – Unsaturated hydrocarbons include alkenes (double bonds) and alkynes (triple bonds) ...
... Unsaturated hydrocarbons – Double and triple bonds between carbons – Not every carbon has each of its 4 electrons bonded to 4 different atoms – More chemically reactive than saturated compounds, or alkanes – Unsaturated hydrocarbons include alkenes (double bonds) and alkynes (triple bonds) ...
CH 3
... PHYSICAL PROPERTIES OF HETEROATOMIC ORGANIC COMPOUNDS • Heteroatomic groups like hydroxide (- OH), formyl(- CHO) and caboxyl(-COOH) can join to alkyl groups. • The physical properties of these heteroatomic compounds are different than that of hydrocarbons having the same number of carbon atoms. • D ...
... PHYSICAL PROPERTIES OF HETEROATOMIC ORGANIC COMPOUNDS • Heteroatomic groups like hydroxide (- OH), formyl(- CHO) and caboxyl(-COOH) can join to alkyl groups. • The physical properties of these heteroatomic compounds are different than that of hydrocarbons having the same number of carbon atoms. • D ...
+ → + − NH Acid Carboxylic O2H CN R
... a. Find longest chain with amine group b. Use parent name, change “e” to “amine” c. Identify R groups attached to Nitrogen with an “N” instead of numbering like normal groups. d. Diamines: Add “di” before “amine” e. Ex: ...
... a. Find longest chain with amine group b. Use parent name, change “e” to “amine” c. Identify R groups attached to Nitrogen with an “N” instead of numbering like normal groups. d. Diamines: Add “di” before “amine” e. Ex: ...
Chapter 21: Organic Chemistry
... Section 21.1: Introduction to Organic Chemistry (cont.) Elements like nitrogen (N), hydrogen (H), and oxygen (O) bond to carbon in this fashion. • The most distinguished feature of carbon atoms is their ability to share electrons with other carbon atoms to form covalent carbon-carbon bonds. • Carbo ...
... Section 21.1: Introduction to Organic Chemistry (cont.) Elements like nitrogen (N), hydrogen (H), and oxygen (O) bond to carbon in this fashion. • The most distinguished feature of carbon atoms is their ability to share electrons with other carbon atoms to form covalent carbon-carbon bonds. • Carbo ...
Carbonyls
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Organic Chemistry II Introduction
... Addition of HCN yields cyanohydrins 1° amines add to form imines, and 2° amines yield enamines Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes a ...
... Addition of HCN yields cyanohydrins 1° amines add to form imines, and 2° amines yield enamines Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes a ...
Basic Organic Chemistry Laboratory Course
... test tube add 6 mL of Lucas reagent. Mix the solutions well and leave them to rest. In the test tube containing the tertiary alcohol, an emulsion or two separate layers form almost immediately. The secondary alcohol reacts more slowly. An emulsion or different layers appear after 510 minutes. The ...
... test tube add 6 mL of Lucas reagent. Mix the solutions well and leave them to rest. In the test tube containing the tertiary alcohol, an emulsion or two separate layers form almost immediately. The secondary alcohol reacts more slowly. An emulsion or different layers appear after 510 minutes. The ...
103. Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling
... substrates containing sensitive functional groups. Fortunately, it was found that the preformed Cs salts of the starting acids were also competent in the reaction (entry 5). In contrast to the parent acid (and, indeed, to most activating groups for tertiary alcohols used for radical generation),2,3 ...
... substrates containing sensitive functional groups. Fortunately, it was found that the preformed Cs salts of the starting acids were also competent in the reaction (entry 5). In contrast to the parent acid (and, indeed, to most activating groups for tertiary alcohols used for radical generation),2,3 ...
Amines
... water-insoluble organic compounds l The amine is extracted into aqueous acid l The amine is recovered by making the solution basic and extracting the amine into an organic solvent t Amides are not basic and are not soluble in aqueous acids ...
... water-insoluble organic compounds l The amine is extracted into aqueous acid l The amine is recovered by making the solution basic and extracting the amine into an organic solvent t Amides are not basic and are not soluble in aqueous acids ...
Organic Chemistry Notes
... .attached F, Cl Br or I atoms called: fluoro, chloro, bromo and iodo groups .use number to indicate attachment position on hydrocarbon chain .if more than one of same kind of halogen is present, use prefixes di, tri, etc. .if compound contains both alkyl and halo groups, list attached groups in alph ...
... .attached F, Cl Br or I atoms called: fluoro, chloro, bromo and iodo groups .use number to indicate attachment position on hydrocarbon chain .if more than one of same kind of halogen is present, use prefixes di, tri, etc. .if compound contains both alkyl and halo groups, list attached groups in alph ...
Organic Chemistry
... Indicate how many carbon atoms are in the entire molecule You will only see molecules with a max of 10 carbons ...
... Indicate how many carbon atoms are in the entire molecule You will only see molecules with a max of 10 carbons ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
Nucleophilic Additions to Carbonyl Group
... group itself. Thus, even a nucleophile that is not very reactive with a carbonyl reacts readily with the conjugate acid. Of the seven functional groups containing a carbonyl group, only aldehydes and ketones commonly undergo nucleophilic addition. The remaining five carbonyl functional groups underg ...
... group itself. Thus, even a nucleophile that is not very reactive with a carbonyl reacts readily with the conjugate acid. Of the seven functional groups containing a carbonyl group, only aldehydes and ketones commonly undergo nucleophilic addition. The remaining five carbonyl functional groups underg ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.