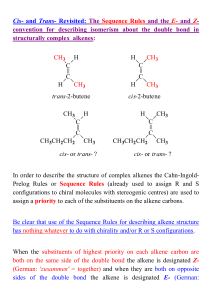
Chapter 1--Title - Chemistry Workshop
... The azo coupling results in compounds which are highly conjugated and which often absorb light in the visible region The -SO3-Na+ group is added to the molecule to confer water solubility and to link the dye to the polar fibers of wool, cotton etc. Orange II is made from 2-naphthol ...
... The azo coupling results in compounds which are highly conjugated and which often absorb light in the visible region The -SO3-Na+ group is added to the molecule to confer water solubility and to link the dye to the polar fibers of wool, cotton etc. Orange II is made from 2-naphthol ...
CHEM 263 Oct 14 revised
... Isopropanol dissociates to from isopropoxide anion and a proton. However, the dissociation constant is 1 x 10-18. Most of the molecules exist in the isopropanol form and only one part in 1018 is ionized. The equilibrium for this reaction lies far to the left. There are two alkyl groups attached to t ...
... Isopropanol dissociates to from isopropoxide anion and a proton. However, the dissociation constant is 1 x 10-18. Most of the molecules exist in the isopropanol form and only one part in 1018 is ionized. The equilibrium for this reaction lies far to the left. There are two alkyl groups attached to t ...
ppt
... intermediate with formal opposite charges on adjacent atoms (overall charge neutral). R4 ...
... intermediate with formal opposite charges on adjacent atoms (overall charge neutral). R4 ...
Alcohol
... The OH group is not a good leaving group in nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However, if the oxygen is first protonated to give R−OH2+, the leaving group (water) is much more stable, and the nucleophilic substitution can take place. For instan ...
... The OH group is not a good leaving group in nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However, if the oxygen is first protonated to give R−OH2+, the leaving group (water) is much more stable, and the nucleophilic substitution can take place. For instan ...
organic compound containing nitrogen
... alkyl groups are attached to nitrogen. Here all four sp3 orbitals are used to form bonds and quatenary nitrogen is tetrahedral. Thus quaternary ammonium salts in which nitrogen holds four different groups have been found to exist as configurational enantiomers, capable of showing optical activity. C ...
... alkyl groups are attached to nitrogen. Here all four sp3 orbitals are used to form bonds and quatenary nitrogen is tetrahedral. Thus quaternary ammonium salts in which nitrogen holds four different groups have been found to exist as configurational enantiomers, capable of showing optical activity. C ...
Chapter 18 – Carbonyl Compounds II (Last Chapter we mostly talk
... removed is actually quite useful in organic chemistry. For example if we wanted to do the following transformation…) 2. Example in Synthesis… ...
... removed is actually quite useful in organic chemistry. For example if we wanted to do the following transformation…) 2. Example in Synthesis… ...
Class 15
... 3) Finish with -amine as the suffix (does NOT take priority over -ol, -ene, or -yne) ...
... 3) Finish with -amine as the suffix (does NOT take priority over -ol, -ene, or -yne) ...
Drawing Organic Structures Functional Groups
... • Change “-oic acid” to “-amide” • Examples: Structure ...
... • Change “-oic acid” to “-amide” • Examples: Structure ...
Organic Nomenclature
... Smaller than five carbons, very reactive. Rings of carbon atoms. Isomers ...
... Smaller than five carbons, very reactive. Rings of carbon atoms. Isomers ...
Alkenes notes
... The fact that alkenes can react by heterolytic mechanisms, however, does not mean that the -bond will not undergo homolytic fission as well. On the contrary - since the bond is non-polar it is very likely to undergo homolytic fission. Alkenes can thus react in two ways: - by free radical addition - ...
... The fact that alkenes can react by heterolytic mechanisms, however, does not mean that the -bond will not undergo homolytic fission as well. On the contrary - since the bond is non-polar it is very likely to undergo homolytic fission. Alkenes can thus react in two ways: - by free radical addition - ...
4.9 Preparation of Alkyl Halides from Alcohols and Hydrogen Halides
... A mixture of sodium bromide and sulfuric acid may be used in place of HBr. ...
... A mixture of sodium bromide and sulfuric acid may be used in place of HBr. ...
Alkenes 3 - ChemWeb (UCC)
... indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the actual mechanism of the elimination process. Notice that the requirement ...
... indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the actual mechanism of the elimination process. Notice that the requirement ...
Basic definitions for organic chemistry
... Organic chemistry is a vast subject so is split it into small sections for study. This is done by studying compounds which behave in a similar way because they have a particular atom, or group of atoms, (FUNCTIONAL GROUP) in their structure. ...
... Organic chemistry is a vast subject so is split it into small sections for study. This is done by studying compounds which behave in a similar way because they have a particular atom, or group of atoms, (FUNCTIONAL GROUP) in their structure. ...
Common aldehydes and ketones
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
pptx
... the skilled experimentalist to stop at the alkene. This is a particularly good way to generate cis double bonds. Note that the Wittig reaction also gives cis alkenes (usually), but most other olefinations give trans products. Therefore partial reduction from an alkyne can be a useful strategy! ...
... the skilled experimentalist to stop at the alkene. This is a particularly good way to generate cis double bonds. Note that the Wittig reaction also gives cis alkenes (usually), but most other olefinations give trans products. Therefore partial reduction from an alkyne can be a useful strategy! ...
Presentation8_108
... completely miscible with water. The solubility rapidly decreases with increase in molecular mass. The higher members are almost insoluble in water but are soluble in organic solvents like benzene, ether etc. The solubility of lower alcohols is due to the existence of hydrogen bonds between water a ...
... completely miscible with water. The solubility rapidly decreases with increase in molecular mass. The higher members are almost insoluble in water but are soluble in organic solvents like benzene, ether etc. The solubility of lower alcohols is due to the existence of hydrogen bonds between water a ...
Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to
... stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained ...
... stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained ...
Silica supported zinc chloride catalyzed acetylation of amines
... revealed that the application of inorganic solids especially zeolites have recently received considerable attention due to their unique physical and chemical properties. H-FER20 has been reported for the acetylation of alcohols and phenols under solventfree conditions. However, some of these acetyla ...
... revealed that the application of inorganic solids especially zeolites have recently received considerable attention due to their unique physical and chemical properties. H-FER20 has been reported for the acetylation of alcohols and phenols under solventfree conditions. However, some of these acetyla ...
chapter 5: nomenclature
... The next alkane contains three carbons, the next one four carbons, the next one five carbons, and so on. The names arise directly from the number of carbons. A couple of important points to note: All the names end in ane - thus the alkanes. There is no other way around this-you must learn the name ...
... The next alkane contains three carbons, the next one four carbons, the next one five carbons, and so on. The names arise directly from the number of carbons. A couple of important points to note: All the names end in ane - thus the alkanes. There is no other way around this-you must learn the name ...
Chapter 17
... While a Fischer esterification is an example of reacting a carboxylic acid in acidic conditions by first protonating the carbonyl, most basic nucleophiles will simply deprotonate the ...
... While a Fischer esterification is an example of reacting a carboxylic acid in acidic conditions by first protonating the carbonyl, most basic nucleophiles will simply deprotonate the ...
CHE2060 Syllabus - Joan`s courses
... No late work will be accepted. However, acknowledging that we can all have a bad day, I will drop one zero for the semester. In-class examples are not collected or graded. Homework problems are due at the next class meeting (2 days or weekend). Lab reports are due at our next lab meeting (1 ...
... No late work will be accepted. However, acknowledging that we can all have a bad day, I will drop one zero for the semester. In-class examples are not collected or graded. Homework problems are due at the next class meeting (2 days or weekend). Lab reports are due at our next lab meeting (1 ...
Organic Molecules
... This idea of a mystical 'vital force' in organic compounds was weakened when scientists began to manufacture organic compounds in the laboratory from non-living materials. One of the rst to do this was Friedrich Wohler in 1828, who successfully prepared urea, an organic compound in the urine of ani ...
... This idea of a mystical 'vital force' in organic compounds was weakened when scientists began to manufacture organic compounds in the laboratory from non-living materials. One of the rst to do this was Friedrich Wohler in 1828, who successfully prepared urea, an organic compound in the urine of ani ...
A Brief History of Organic Chemistry
... derivatives. These compounds contain carbon and some other non-hydrogen element usually a nonmetal. Most, but not all hydrocarbon derivatives also contain hydrogen. You are already familiar with many hydrocarbon derivatives. Gas-line antifreeze (methanol), vinegar (acetic acid), amino acids, and sug ...
... derivatives. These compounds contain carbon and some other non-hydrogen element usually a nonmetal. Most, but not all hydrocarbon derivatives also contain hydrogen. You are already familiar with many hydrocarbon derivatives. Gas-line antifreeze (methanol), vinegar (acetic acid), amino acids, and sug ...
Types of Functional Groups Amines
... of carbons takes the root name and the other chains become a substituents located on the N When the three alkyl groups are the same it can be named as a trialkyl amine Ex: trimethyl amine ...
... of carbons takes the root name and the other chains become a substituents located on the N When the three alkyl groups are the same it can be named as a trialkyl amine Ex: trimethyl amine ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.