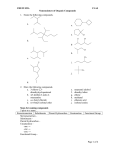
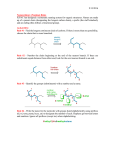
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Drawing Organic Structures Functional Groups
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Aromatization wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aromaticity wikipedia , lookup
Asymmetric induction wikipedia , lookup
NOMENCLATURE Dr. Clower CHEM 2411 Spring 2014 McMurry (8th ed.) sections 3.2, 3.3, 3.4, 4.1, 4.2, 7.3, 7.4, 9.1, 10.1, 15.1, 17.1,18.1, 18.8, 19.1, 20.1, 21.1, 24.1, Appendix A Nomenclature • The naming of organic molecules • IUPAC system • All names have Substituents-MainChain • Substituents are groups attached to the main chain • MainChain consists of Parent-Infix-Suffix • Parent tells you number of carbons in main chain • Infix tells you if the carbon-carbon bonds are single bonds (saturated molecule) or double or triple bonds (unsaturated molecules) • Suffix tells you the functional group of your molecules Parent Number of carbons Parent name 1 meth- 2 eth- 3 prop- 4 but- 5 pent- 6 hex- 7 hept- 8 oct- 9 non- 10 dec- 11 undec- 12 dodec- Straight-chain alkanes Infix • “-an-” = only carbon-carbon single bonds (saturated) • “-en-” = an alkene (double bond) is present (unsaturated) • “-yn-” = an alkyne (triple bond) is present (unsaturated) Suffix Functional group Suffix alcohol -ol ether ether amine -amine nitrile -nitrile thiol -thiol sulfide sulfide aldehyde -al ketone -one carboxylic acid -oic acid ester -oate amide -amide acid chloride -oyl chloride acid anhydride -oic anhydride Nomenclature • Consider the molecule 2-hexanol. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • Consider the molecule octanoic acid. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • Consider the molecule 4-methyl-2-pentanamine. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • What is 4-methyl? Substituents • Groups off of main chain (branches) • Parts of larger compounds (not stable by themselves) • Alkyl groups = Alkane – H • “-ane” changes to “-yl” • Methyl • methane – H • CH4 – H • CH3─ • Ethyl • ethane – H • CH3CH3 – H • CH3CH2─ Substituents • Propane – H gives two possible alkyl groups • Propyl • Isopropyl • Similarly, there are multiple alkyl groups with 4 carbon atoms, 5 carbon atoms, etc. Alkyl groups you need to know • Methyl • Pentyl (amyl) • Ethyl • Isopentyl (isoamyl) • Propyl • Neopentyl • Isopropyl • tert-pentyl • Butyl • Hexyl, heptyl, etc. • Isobutyl • Halogens • Fluoro • Chloro • Bromo • Iodo • sec-butyl • tert-butyl (t-butyl) Naming alkanes 1. Find the parent chain • Longest continuous chain of C atoms • Name of chain = parent name • If 2 chains of equal length, parent is the chain with more substituents 2. Number each carbon in the parent chain • Start from the end closest to the first substituent • If there are substituents equal distance from both ends, number from the end nearest the second substituent Naming alkanes, cont. 3. Name and number substituents • Name = alkyl group name • Number = point of attachment to parent chain • Two substituents on the same C get the same number 4. Write the name as a single word • Substituents before parent name (include #) • Separate # and word with hyphen • Separate two numbers with a comma • List substituents in alphabetical order • Multiple identical substituents use prefixes di, tri, tetra, penta, hexa • DO NOT use sec, tert, di, tri, etc. when alphabetizing • DO use iso, neo when alphabetizing Examples Structure Name Examples • Draw 3-ethylpentane. • Draw all the constitutional isomers with the molecular formula C6H14. Name each isomer. Examples Structure Name Examples Structure Name Naming Cycloalkanes • Unsubstituted cycloalkanes: • Add “cyclo” to the parent name • Bicycloalkanes • Two rings • Most common is norbornane • Bicyclo[2.2.1]heptane Naming Cycloalkanes • Substituted cycloalkanes: • Parent = ring or substituent (whichever has more carbons) • Number the substituents • Do not need to show number if only one substituent on a ring • If two substituents, start with the first alphabetically, number in the direction of the second substituent • If more than two substituents, number so that the substituents have the lowest set of numbers Examples Structure Name Examples Structure Name Cycloalkane Stereoisomers • Cycloalkanes are roughly planar • Substituents are either above or below this plane • Shown with dash and wedges • Dash = back; wedge = forward • In a disubstituted cycloalkane, two substituents on the same side (both back or both forward) are cis. Two substituents on opposite sides (one back, one forward) are trans. • Cis and trans versions of the same molecule are stereoisomers Examples Structure Name • Do these pairs represent constitutional isomers, cis- trans stereoisomers or the same compound? (a) (b) Alkene Nomenclature • Similar to alkanes • Change infix from “-an-” to “-en-” Naming Alkenes • For larger alkenes: 1. Parent is longest C chain containing both carbons of C=C 2. Number chain so C=C has lowest possible number • If the double bond is equidistant from both ends, start numbering at end nearest the first substituent • Show location of C=C by first number • Alkenes with >1 C=C use “-adiene”, “-atriene”, etc. in place of “-ene” and show location of all double bonds 3. Name and number substituents and write the full name • Example: Examples Structure Name Cycloalkenes • The carbon atoms of the C=C are numbered 1 and 2 • Number ring in direction to give first substituent lowest possible number Structure Name Alkene substituents Substituent Name CH2═ methylene CH2═CH─ vinyl CH2═CH─CH2─ allyl Alkene Stereoisomers • Cis-trans stereoisomers • Seen with disubstituted alkenes • Cis means groups are on the same side of the double bond; trans means groups are on opposite sides • Cannot convert through bond rotation • Example: 2-butene Examples • Are the following alkenes cis, trans, or neither? (a) (b) (c) Alkyne Nomenclature • Similar to alkenes • Change infix from “-en-” to “-yn-” Naming Alkynes • For larger alkynes: 1. Parent is longest C chain containing both carbons of C≡C 2. Number chain so C≡C has lowest possible number • If the triple bond is equidistant from both ends, start numbering at end nearest the first substituent • Show location of C≡C by first number • Alkynes with >1 C≡C use “-adiyne”, “-atriyne”, etc. in place of “-yne” and show location of all double bonds 3. Name and number substituents and write the full name • Example: Enyne • Contains both alkene and alkyne • Number from end closest to first multiple bond (either C=C or C≡C) and show both numbers • If the C=C and C≡C are equidistant from the ends, C=C gets the lower number • Examples: Structure Name Naming Aromatic Compounds • Benzene • Monosubstituted benzenes • Substituent name + “benzene” Common Benzene Compounds Benzene Nomenclature • If substituent has greater than 6 carbons, it becomes the parent, and benzene is called a phenyl group • Benzene substituents: Disubstituted Benzenes • ortho (1,2) • meta (1,3) • para (1,4) Naming Disubstituted Benzenes • If one substituent is part of a common name, that name is the parent and that substituent is at carbon 1 • If neither substituent is part of a common name, list the substituents in alphabetical order (first alphabetically is at carbon 1) • If both substituents are part of common name, use this order of priority to determine the parent name: -CO2H > -CHO > -OH > -NH2 > -CH3 Examples Structure Name Examples Structure Name Naming Polysubstituted Benzenes • With 3 or more substituents do not use ortho, meta, para • Number ring to give smallest set of numbers • If a common name, use as parent (substituent at carbon 1) • List substituents in alphabetical order Examples Structure Name Another Aromatic Compound • Pyridine • If substituted, nitrogen is atom 1 of the ring. Number in direction of other substituents. Naming Alcohols • Acyclic alcohols 1. Parent chain is longest chain containing C bonded to –OH 2. Change suffix from “-e” to “-ol” 3. Number from end closest to –OH. Show location of –OH. 4. Name/number substituents • Cyclic alcohols 1. Ring is the parent 2. Number ring so –OH is at carbon 1 and other substituents have lowest possible numbers. You do not need to show the location of the –OH. 3. Name/number substituents. Naming Alcohols • Multiple hydroxyl groups • Two –OH groups is a diol; 3 is a triol • Two adjacent –OH groups is a glycol • Name as acyclic alcohols, except keep the “-e” suffix and add “-diol” • Indicate numbers for all –OH groups • Unsaturated alcohols (enol or ynol) 1. Parent chain contains carbon bonded to –OH and both carbons of C=C or C≡C 2. Suffix is “-ol”, infix is “-en-” or “-yn-” 3. Number chain so –OH has the lowest number 4. Show numbers for –OH and the unsaturation 5. Name/number substituents Examples Structure Name Examples Structure Name Naming Thiols • Thiols are sulfur analogs of alcohols • Name like alcohols, but keep the “-e” and use “-thiol” in place of “-ol” Naming Amines 1. Parent chain is longest containing C bonded to –N 2. Change suffix “-e” to “-amine” 3. Number from end closest to –N. Show location of –N. 4. Name/number substituents Examples Structure Name Naming Aldehydes • Parent chain contains carbon of CHO • Suffix is “-al” • CHO carbon is carbon 1 (do not need to show in name) Naming Aldehydes • Cyclic molecules with –CHO substituents • -CHO is bonded to carbon 1 of ring • Add “carbaldehyde” to end of ring parent name Naming Ketones • Parent chain contains carbon of carbonyl; suffix is “-one” • Number so carbonyl has lowest number • Cyclic ketones carbonyl is carbon 1 of the ring • Some more common names: Examples Structure Name Order of Precedence of Functions • Used when more than one functional group in a Increasing precedence molecule Functional Group Suffix (High Precedence) Prefix (Low Precedence) -CO2H -oic acid - -CHO -al formyl- -C(O)- -one oxo- -OH -ol hydroxy- -NH2 -amine amino- Examples Structure Name Naming Carboxylic Acids • Parent chain contains carbon of –CO2H • Suffix is “-oic acid” • –CO2H is carbon 1 Naming Carboxylic Acids • Cyclic molecules with –CO2H substituents • –CO2H is bonded to carbon 1 of ring • Add “carboxylic acid” to end of ring parent name Examples Structure Name Naming Acid Chlorides • Name corresponding carboxylic acid • Change “-ic acid” to “-yl chloride” • Examples: Structure Name Naming Acid Anhydrides • If R = R’, name carboxylic acid RCO2H. Replace “acid” with “anhydride” • If R ≠ R’, list the two acids alphabetically and add the word “anhydride” • Examples: Structure Name Naming Esters • Name alkyl group bonded to oxygen (R’) • Name carboxylic acid RCO2H • Change “-ic acid” to “-ate” • Examples: Structure Name Naming Amides • Name corresponding carboxylic acid • Change “-oic acid” to “-amide” • Examples: Structure Name Example • DMF (N,N-dimethylformamide) is a common solvent in organic chemistry. Draw the structure of DMF. Naming Nitriles • Two methods 1. Nitrile carbon is carbon 1 of parent chain. Add “-nitrile” to end of alkane name. 2. Name as carboxylic acid derivative. Replace “-ic acid” with “-onitrile” Common Name System • Can be used for some simple molecules • Alkyl halides, alcohols, amines • Name alkyl group • Add “chloride” or “bromide” or “alcohol” or “amine” • Examples: Structure CH3Cl (CH3)2CHBr CH3CH2OH CH3CH2NHCH3 (CH3CH2)2NH Name Common Name System • Ketones, ether • Name both alkyl groups bonded to carbonyl (ketone), oxygen (ether), or sulfur (sulfide) • Add “ketone” or “ether” or “sulfide” • Examples: Structure Name