friedel-craft reaction: a review - Advance Institute of Biotech
... Friedel–Crafts alkylation of arenes with ketones and N-arylsulfonyl aldimines as alkylation reagents were observed. In a few cases, aromatic aldehydes were also found to be used in the Friedel–Crafts alkylation, especially for electron-rich arenes and aromatic heterocyclic compounds, such as phenols ...
... Friedel–Crafts alkylation of arenes with ketones and N-arylsulfonyl aldimines as alkylation reagents were observed. In a few cases, aromatic aldehydes were also found to be used in the Friedel–Crafts alkylation, especially for electron-rich arenes and aromatic heterocyclic compounds, such as phenols ...
notes07
... were observed at all for this system! The problem with these experiments is that it is almost impossible to do the experiments without at least some H2O present. And, as it turns out, even a little (approx. 10 ppm) water will accelerate the oxidation of CO dramatically. In fact, in just about all co ...
... were observed at all for this system! The problem with these experiments is that it is almost impossible to do the experiments without at least some H2O present. And, as it turns out, even a little (approx. 10 ppm) water will accelerate the oxidation of CO dramatically. In fact, in just about all co ...
Working with Hazardous Chemicals
... 7. Chlorine should be introduced slowly at first to prevent an accumulation of unreacted chlorine in the solution and avoid the risk of a rapid, exothermic reaction. The accumulation of chlorine is indicated by the appearance of its characteristic yellow-green color. 8. The checkers used a 15 × 7.5 ...
... 7. Chlorine should be introduced slowly at first to prevent an accumulation of unreacted chlorine in the solution and avoid the risk of a rapid, exothermic reaction. The accumulation of chlorine is indicated by the appearance of its characteristic yellow-green color. 8. The checkers used a 15 × 7.5 ...
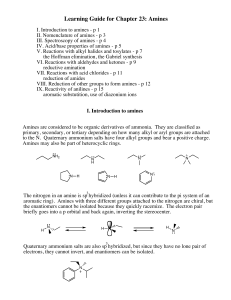
Learning Guide for Chapter 23: Amines
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... Quaternary Ammonium Salts (R4N+ X-) Nitrogen atoms are positively charged when bonded to four substituents: when all of these substituents are carbon, the molecule is referred to as a quaternary ammonium ion. ...
... Quaternary Ammonium Salts (R4N+ X-) Nitrogen atoms are positively charged when bonded to four substituents: when all of these substituents are carbon, the molecule is referred to as a quaternary ammonium ion. ...
ch18 by dr. dina
... The carboxyl proton of most carboxylic acids has a pKa = 4 - 5 Carboxylic acids are readily deprotonated by sodium hydroxide or sodium bicarbonate to form carboxylate salts Carboxylate salts are more water soluble than the corresponding carboxylic acid ...
... The carboxyl proton of most carboxylic acids has a pKa = 4 - 5 Carboxylic acids are readily deprotonated by sodium hydroxide or sodium bicarbonate to form carboxylate salts Carboxylate salts are more water soluble than the corresponding carboxylic acid ...
Final Sc. Ed. 423. Chemistry II
... Describe isomerism and physical properties of aldehydes and ketones. Describe the structure of carbonyl group. Explain the reactions of carbonyl compounds with HCN and its mechanism, NaHSO3, RMgX, alcohols with mechanism, ammonia derivatives with mechanism Explain Aldol condensation, Canniza ...
... Describe isomerism and physical properties of aldehydes and ketones. Describe the structure of carbonyl group. Explain the reactions of carbonyl compounds with HCN and its mechanism, NaHSO3, RMgX, alcohols with mechanism, ammonia derivatives with mechanism Explain Aldol condensation, Canniza ...
ch04 - alkanes
... Alkanes= saturated hydrocarbons Chapter 4 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis ...
... Alkanes= saturated hydrocarbons Chapter 4 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis ...
Chemistry in Action: Question paper - A
... (b) The students deduced that the heat change was due only to the formation of intermolecular forces between ethyl ethanoate molecules and trichloromethane molecules. Ignoring all experimental errors, give one reason why the students may have made an incorrect deduction. ...
... (b) The students deduced that the heat change was due only to the formation of intermolecular forces between ethyl ethanoate molecules and trichloromethane molecules. Ignoring all experimental errors, give one reason why the students may have made an incorrect deduction. ...
Organic Chemistry II / CHEM 252 Chapter 16
... • Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate – The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) – The equilibrum favors a ketone over its ...
... • Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate – The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) – The equilibrum favors a ketone over its ...
ORGANIC CHEMISTRY
... 1. Determine the longest continuous chain of carbon. (Parent chain) 2. Number carbons in parent chain. Do this by starting at the end that gives the attached groups the smallest number. 3. Add numbers to the names of the attached groups to identify their position. Numbers become prefixes to the name ...
... 1. Determine the longest continuous chain of carbon. (Parent chain) 2. Number carbons in parent chain. Do this by starting at the end that gives the attached groups the smallest number. 3. Add numbers to the names of the attached groups to identify their position. Numbers become prefixes to the name ...
Faculteit der Natuurwetenschappen, Wiskunde en Informatica
... diastereomeric ratios are obtained; from 55:45 to 93:7. It should be noted that using bulkier Grignard reagents in the addition leads to higher diastereomeric ratios. This is most likely because bulkier substituents are more sterically hindered and causes the Grignard reagent to only attack the side ...
... diastereomeric ratios are obtained; from 55:45 to 93:7. It should be noted that using bulkier Grignard reagents in the addition leads to higher diastereomeric ratios. This is most likely because bulkier substituents are more sterically hindered and causes the Grignard reagent to only attack the side ...
cleavage of methyl ethers with iodotrimethylsilane
... prepared by the procedure described in the following paragraph. The submitters have used chlorotrimethylsilane purchased from Aldrich Chemical Company, Inc., and Silar Laboratories, Inc. (10 Alplaus Road, Scotia, New York 12302) either as supplied or after distillation from calcium hydride. No appre ...
... prepared by the procedure described in the following paragraph. The submitters have used chlorotrimethylsilane purchased from Aldrich Chemical Company, Inc., and Silar Laboratories, Inc. (10 Alplaus Road, Scotia, New York 12302) either as supplied or after distillation from calcium hydride. No appre ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... halides to form tri- or tetra ortho-substitutedbiaryls was first examined using the conditions previously reported by Buchwald and coworkers. 2 0 Initial experiments showed that this system did not give any of the desired cross-coupling products, hence a number of different biaryl phosphine ligands ...
... halides to form tri- or tetra ortho-substitutedbiaryls was first examined using the conditions previously reported by Buchwald and coworkers. 2 0 Initial experiments showed that this system did not give any of the desired cross-coupling products, hence a number of different biaryl phosphine ligands ...
Branched-Chain Alkanes
... Unsaturated Compounds: Alkenes and Alkynes • have fewer hydrogen atoms attached to the carbon chain than alkanes • are alkenes with C=C double bonds • are alkynes with triple C≡C bonds ...
... Unsaturated Compounds: Alkenes and Alkynes • have fewer hydrogen atoms attached to the carbon chain than alkanes • are alkenes with C=C double bonds • are alkynes with triple C≡C bonds ...
SULFONATION OF BY SO3
... The sulfonation process relates to the sulfur trioxide sulfonation of long chain and branched chain aliphatic alcohols such as fatty alcohols, and to the sulfonation of the aromatic nucleus of alkyl aromatic compounds. The reaction of sulfur trioxide is with an alkyl benzene whereby the sulfonated a ...
... The sulfonation process relates to the sulfur trioxide sulfonation of long chain and branched chain aliphatic alcohols such as fatty alcohols, and to the sulfonation of the aromatic nucleus of alkyl aromatic compounds. The reaction of sulfur trioxide is with an alkyl benzene whereby the sulfonated a ...
Chemistry 1110 – Organic Chemistry IUPAC Nomenclature
... Chemistry 1110 – Organic Chemistry IUPAC Nomenclature Of the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPAC system of nomenclature was established at the end of the 19th century in order for chemis ...
... Chemistry 1110 – Organic Chemistry IUPAC Nomenclature Of the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPAC system of nomenclature was established at the end of the 19th century in order for chemis ...
Chapter 11: Reactions of Alcohols
... converted into a group that is a weaker base (and therefore a better leaving group), a nucleophilic substitution reaction can occur. One way to convert an OH group into a weaker base is to protonate it by adding acid to the solution. ...
... converted into a group that is a weaker base (and therefore a better leaving group), a nucleophilic substitution reaction can occur. One way to convert an OH group into a weaker base is to protonate it by adding acid to the solution. ...
SYNOPSIS
... heterocycles such as pyroles and indoles. The analogous reaction of indoles with other aromatic or aliphatic aldehyde and ketones produces azafulvenium salts. The azafulvenium salts can undergo further addition with the second indole molecule to afford bis (indolyl) methanes. Protic acids as well as ...
... heterocycles such as pyroles and indoles. The analogous reaction of indoles with other aromatic or aliphatic aldehyde and ketones produces azafulvenium salts. The azafulvenium salts can undergo further addition with the second indole molecule to afford bis (indolyl) methanes. Protic acids as well as ...
PDF document
... above all in carbon-carbon bond formation, mainly through metal catalysed coupling reactions. In addition, many iodinated aromatic derivatives are used in medicine as drugs or diagnostic aids, contrasting agents, and radioactively labelled markers. The chemistry dealing with selective iodination of ...
... above all in carbon-carbon bond formation, mainly through metal catalysed coupling reactions. In addition, many iodinated aromatic derivatives are used in medicine as drugs or diagnostic aids, contrasting agents, and radioactively labelled markers. The chemistry dealing with selective iodination of ...
Carbonyl Compounds - Thomas Tallis Science
... Like alkenes, ketones with four or more carbon atoms display positional isomerism because the carbonyl group may appear between different carbon atoms. In these cases, a number is used before the –one to indicate the first carbon ...
... Like alkenes, ketones with four or more carbon atoms display positional isomerism because the carbonyl group may appear between different carbon atoms. In these cases, a number is used before the –one to indicate the first carbon ...
Chlorotrimethylsilane/Sodium Iodide, a
... Isolated yield. T h e products were characterized by comparing IR, N M R , a n d b p or m p with those of the authentic samples. 10% of N-(1-adamanty1)acetamidewas also isolated in this experiment. c Cholesterol was solubilized using a mixture of chloroform a n d acetonitrile as the sohent. mmol) an ...
... Isolated yield. T h e products were characterized by comparing IR, N M R , a n d b p or m p with those of the authentic samples. 10% of N-(1-adamanty1)acetamidewas also isolated in this experiment. c Cholesterol was solubilized using a mixture of chloroform a n d acetonitrile as the sohent. mmol) an ...
Organic Spectroscopy UV - Ultraviolet-Visible Spectroscopy
... Ethene has lmax at 171 nm and 1,3-butadiene has lmax at 217 nm The longer the conjugated system, the smaller the energy difference between the HOMO and the LUMO A smaller energy gap results in longer !max in the ultraviolet -visible spectrum #-Carotene has 11 conjugated double bonds and an absorbanc ...
... Ethene has lmax at 171 nm and 1,3-butadiene has lmax at 217 nm The longer the conjugated system, the smaller the energy difference between the HOMO and the LUMO A smaller energy gap results in longer !max in the ultraviolet -visible spectrum #-Carotene has 11 conjugated double bonds and an absorbanc ...
Exam 2
... Chern 24 2 (w 2016) exam #2B 1. (10 pts) Circle what is true about Substitution and elimination reactions. ...
... Chern 24 2 (w 2016) exam #2B 1. (10 pts) Circle what is true about Substitution and elimination reactions. ...
Carbonyl α-substitution and Condensation Reactions
... Amines are organic compounds derived from ammonia with one or more alkyl groups bonded to nitrogen. The chemistry of amines is dominated by the lone pair of electrons on the nitrogen. The lone pair of electrons on the nitrogen of amines is a powerful electron source, so the most important chemical p ...
... Amines are organic compounds derived from ammonia with one or more alkyl groups bonded to nitrogen. The chemistry of amines is dominated by the lone pair of electrons on the nitrogen. The lone pair of electrons on the nitrogen of amines is a powerful electron source, so the most important chemical p ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.