Organic Compounds: Alkanes
... Organic compounds tend rather to dissolve in organic solvents which are either pure substances like ether or ethyl alcohol, or mixtures, such as the paraffinic solvents such as the various petroleum. Like inorganic salts, organic compounds may also form crystals. Unique property of carbon in organic ...
... Organic compounds tend rather to dissolve in organic solvents which are either pure substances like ether or ethyl alcohol, or mixtures, such as the paraffinic solvents such as the various petroleum. Like inorganic salts, organic compounds may also form crystals. Unique property of carbon in organic ...
carboxylic acids and their derivatives
... indicated by the suffix -oic with the addition of the word acid. When the carbon atom of the COOH group is not a part of the parent structure, the ending -carboxylic acid is added (see Example 5 in Chapter 2). Acyl groups RC(O)- are named from the corresponding acids by changing the suffix -ic of th ...
... indicated by the suffix -oic with the addition of the word acid. When the carbon atom of the COOH group is not a part of the parent structure, the ending -carboxylic acid is added (see Example 5 in Chapter 2). Acyl groups RC(O)- are named from the corresponding acids by changing the suffix -ic of th ...
The Carboxylic Acid Group as an Effective Director of Ortho
... redistilled (bp 120.5-121.0 "C) NJV,N',"-tetramethyl-l,2-ethylenediamine and 100 mL of dry THF. The mixture was stirred magnetically and cooled to -90 "C. After consecutive addition of sec-butyllithium (0.110 mol, 80 mL of 1.4 M cyclohexanehexanes solution) and recrystallized benzoic acid (mp 122-12 ...
... redistilled (bp 120.5-121.0 "C) NJV,N',"-tetramethyl-l,2-ethylenediamine and 100 mL of dry THF. The mixture was stirred magnetically and cooled to -90 "C. After consecutive addition of sec-butyllithium (0.110 mol, 80 mL of 1.4 M cyclohexanehexanes solution) and recrystallized benzoic acid (mp 122-12 ...
Chapter 16
... In Chapter 15, it was shown how there is an enormous number of hydrocarbons as a result of the ability of carbon compounds to catenate. These compounds can have structures which are straight chains, branched chains, or rings. The number of possibilities is increased many times by the existence of is ...
... In Chapter 15, it was shown how there is an enormous number of hydrocarbons as a result of the ability of carbon compounds to catenate. These compounds can have structures which are straight chains, branched chains, or rings. The number of possibilities is increased many times by the existence of is ...
Full Article-PDF - UNC
... added dropwise to a solution of diisopropylamine (16.35 g, 0.162 mol.) in 50 mL THF which had been precooled to -78°C. The resulting pale yellow solution was stirred 30 min. Methyl isobutyrate (15.00 g, 0.147 mol) in 50 mL THF was added dropwise over 30 min and the light yellow solution was stirred ...
... added dropwise to a solution of diisopropylamine (16.35 g, 0.162 mol.) in 50 mL THF which had been precooled to -78°C. The resulting pale yellow solution was stirred 30 min. Methyl isobutyrate (15.00 g, 0.147 mol) in 50 mL THF was added dropwise over 30 min and the light yellow solution was stirred ...
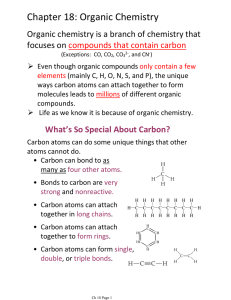
Chapter 18: Organic Chemistry
... nearest the first alkyl substituent. 3. Use the numbers obtained by the application of rule #2 to designate the locations of the alkyl substituent groups. Name the alkyl groups. 4. List alkyl groups alphabetically, along with the location number, before the parent chain name (i.e. ethyl before ...
... nearest the first alkyl substituent. 3. Use the numbers obtained by the application of rule #2 to designate the locations of the alkyl substituent groups. Name the alkyl groups. 4. List alkyl groups alphabetically, along with the location number, before the parent chain name (i.e. ethyl before ...
Biological Spectroscopy - UCO
... Radical Site Initiation (a-Cleavage) - 1 This is particularly important for ions that contain N or O atoms. Electron pairing occurs by the transfer of an odd electron from the bond alpha to the atom carrying the charge and the transfer of the odd lone pair electron to form a new bond. The remaining ...
... Radical Site Initiation (a-Cleavage) - 1 This is particularly important for ions that contain N or O atoms. Electron pairing occurs by the transfer of an odd electron from the bond alpha to the atom carrying the charge and the transfer of the odd lone pair electron to form a new bond. The remaining ...
Chapter 10 Structure and Synthesis of Alcohols
... ¾ Drop the -e from the alkane name, add -ol. ¾ Number the chain, starting from the end closest to the -OH group. ¾ Number and name all substituents. ...
... ¾ Drop the -e from the alkane name, add -ol. ¾ Number the chain, starting from the end closest to the -OH group. ¾ Number and name all substituents. ...
Alcohols and Carbonyls test
... a hint!!!! Ethanol, will in the presence of strong acids, or when the vapour is passed over aluminium oxide, undergoes dehydration to form ethene. ...
... a hint!!!! Ethanol, will in the presence of strong acids, or when the vapour is passed over aluminium oxide, undergoes dehydration to form ethene. ...
C - Glow Blogs
... a hint!!!! Ethanol, will in the presence of strong acids, or when the vapour is passed over aluminium oxide, undergoes dehydration to form ethene. ...
... a hint!!!! Ethanol, will in the presence of strong acids, or when the vapour is passed over aluminium oxide, undergoes dehydration to form ethene. ...
Guide_to_Life_in_Orgo_Ib
... shoulders - Good preparation is key for doing well. With this in mind, when you look at the course outline over the next few pages, you will see that the semester is split up into separate lessons, and the content we will go through in each lesson; there are also preparatory assignments listed for e ...
... shoulders - Good preparation is key for doing well. With this in mind, when you look at the course outline over the next few pages, you will see that the semester is split up into separate lessons, and the content we will go through in each lesson; there are also preparatory assignments listed for e ...
PP Aldehyde and ketone
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
... 2,4-DINITROPHENYLHYDRAZINE C6H3(NO2)2NHNH2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. ...
... we use microwave method [11-15]. They provide an efficient and useful synthetic method of diverse and complex compounds, as well as small and drug-like heterocycles [16-19]. In a higher product yield than classical chemistry [20-25]. The importance of these processes is underscored by the large numb ...
$doc.title
... They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ring Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel addi1ve, ...
... They are important solvents and synthesis intermediates Phenols contain an OH group connected to a carbon in a benzene ring Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel addi1ve, ...
07. Aldehydes and ketones
... Reaction of “silver mirror” and reaction with Fehling reagent used for identification of aldehyde group. ...
... Reaction of “silver mirror” and reaction with Fehling reagent used for identification of aldehyde group. ...
United States Patent Dolphin et al.
... one general method known to convert meso-tetraphenyl 45 porphyrins into the corresponding chlorins, namely the diimide reduction introduced by Whitlock et aI., "Diimide Reduction of Porphyrins", J. Am. Chern. Soc., 91, 7485-89 (1969). However, the product produced does not have a 13,f:l'-dihydroxy s ...
... one general method known to convert meso-tetraphenyl 45 porphyrins into the corresponding chlorins, namely the diimide reduction introduced by Whitlock et aI., "Diimide Reduction of Porphyrins", J. Am. Chern. Soc., 91, 7485-89 (1969). However, the product produced does not have a 13,f:l'-dihydroxy s ...
Chapter 10 Structure and Synthesis of Alcohols
... 3-Methyl-3-pentanol + methanol 2-Methyl-2-butanol + ethanol 2-Methyl-3-butanol + methanol 2-Methyl-3-pentanol + ethanol ...
... 3-Methyl-3-pentanol + methanol 2-Methyl-2-butanol + ethanol 2-Methyl-3-butanol + methanol 2-Methyl-3-pentanol + ethanol ...
Chapter 19. Aldehydes and Ketones
... Nucleophilic addition of the equivalent of a carbon anion, or carbanion. A carbon–magnesium bond is strongly polarized, so a Grignard reagent reacts for all practical purposes as R : MgX +. ...
... Nucleophilic addition of the equivalent of a carbon anion, or carbanion. A carbon–magnesium bond is strongly polarized, so a Grignard reagent reacts for all practical purposes as R : MgX +. ...
Lab 6
... which the acyl group is the acetyl group. Such reactions are called acetylation reactions. These reactions involve the transfer of an acetyl acyl group to an oxygen atom (i.e., the substitution of an acetyl group for an H atom bonded to oxygen). When alcohols or phenols are acylated, the products ar ...
... which the acyl group is the acetyl group. Such reactions are called acetylation reactions. These reactions involve the transfer of an acetyl acyl group to an oxygen atom (i.e., the substitution of an acetyl group for an H atom bonded to oxygen). When alcohols or phenols are acylated, the products ar ...
synthpp - Knockhardy
... When planning a synthetic route, chemists must consider... • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react ...
... When planning a synthetic route, chemists must consider... • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react ...
Preface (PDF, 24 Pages, 5.7 MB)
... Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publication is protected by Copyright, and p ermission should be obtained from the publisher prior to any prohibited reproduction, storage in a retrieval system, or transmis ...
... Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publication is protected by Copyright, and p ermission should be obtained from the publisher prior to any prohibited reproduction, storage in a retrieval system, or transmis ...
Proceedings of the Indiana Academy of Science
... and toxic solvents, and expensive glassware. Furthermore, their completion requires longer than 50 minutes. Such constraints limit the number of organic synthesis experiments, in which products are isolated, purified, and characterized, that are amenable for use in high school. The student's laborat ...
... and toxic solvents, and expensive glassware. Furthermore, their completion requires longer than 50 minutes. Such constraints limit the number of organic synthesis experiments, in which products are isolated, purified, and characterized, that are amenable for use in high school. The student's laborat ...
Orbitals
... 1.9 million tons of formaldehyde, H2C=O, produced each year for insulation materials and resins 1.5 million tons of acetone, ((CH3)2C=O), produced each year for use as ...
... 1.9 million tons of formaldehyde, H2C=O, produced each year for insulation materials and resins 1.5 million tons of acetone, ((CH3)2C=O), produced each year for use as ...
Chapter 8
... Basicity of Amines • Aliphatic amines have about the same base strength, and are slightly stronger bases than NH3. • Aromatic and heterocyclic aromatic. • amines are considerably weaker bases than aliphatic amines. • Note that while aliphatic amines are weak bases by comparison with inorganic bases ...
... Basicity of Amines • Aliphatic amines have about the same base strength, and are slightly stronger bases than NH3. • Aromatic and heterocyclic aromatic. • amines are considerably weaker bases than aliphatic amines. • Note that while aliphatic amines are weak bases by comparison with inorganic bases ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.