SMK RAJA PEREMPUAN, IPOH
... Candidates should be able to : 16.1 Alkanes, exemplified by ethane 1. explain alkanes as saturated aliphatic hydrocarbons (a) Free radical substitution, eg the effect of 2. explain the construction of the alkane series (straight and chlorination of hydrocarbons in water on the branched) and IUPAC no ...
... Candidates should be able to : 16.1 Alkanes, exemplified by ethane 1. explain alkanes as saturated aliphatic hydrocarbons (a) Free radical substitution, eg the effect of 2. explain the construction of the alkane series (straight and chlorination of hydrocarbons in water on the branched) and IUPAC no ...
(C3H7)3NH[CrO3X],(X=F, Cl), Reagents for Oxidation of
... Keywords: Tripropylammonium Fluorochromate(VI); Tripropylammonium Chlorochromate (VI); Heterogeneous Oxidants; Alumina; Oxidation. ______________________________________________________________________________ INTRODUCTION Oxidation of organic compounds in general, and of alcohols in particular, und ...
... Keywords: Tripropylammonium Fluorochromate(VI); Tripropylammonium Chlorochromate (VI); Heterogeneous Oxidants; Alumina; Oxidation. ______________________________________________________________________________ INTRODUCTION Oxidation of organic compounds in general, and of alcohols in particular, und ...
Organic Chemistry ruba
... Can be formed from the dehydration of secondary alcohols with a catalyst. Propanone is formed from the oxidation of 2propanol using KMnO4 or PCC catalyst.* (c) 2006, Mark Rosengarten ...
... Can be formed from the dehydration of secondary alcohols with a catalyst. Propanone is formed from the oxidation of 2propanol using KMnO4 or PCC catalyst.* (c) 2006, Mark Rosengarten ...
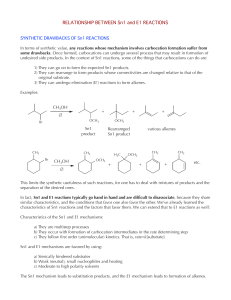
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
Aromatic nitro compounds Background Nomenclature
... also known as nitrobenzol, molecular formula C6H5NO2. Often highly explosive, especially when the compound contains more than one nitro group. One of the most common explosophores (functional group that makes a compound explosive) used globally. ...
... also known as nitrobenzol, molecular formula C6H5NO2. Often highly explosive, especially when the compound contains more than one nitro group. One of the most common explosophores (functional group that makes a compound explosive) used globally. ...
Reactions of Aromatic Compounds
... director than the chlorine atom, and substitution occurs mostly at the positions ortho to the amide. Like an alkoxyl group, the amide is a particularly strong activating group, and the reaction gives some of the dibrominated product. ...
... director than the chlorine atom, and substitution occurs mostly at the positions ortho to the amide. Like an alkoxyl group, the amide is a particularly strong activating group, and the reaction gives some of the dibrominated product. ...
Chap 1 - Notes - StrucandPropOrganicComp
... The difference between cis- and trans- isomers is very important, especially in metabolic processes. Note: triple bonds do not form diastereomers Enantiomers Enantiomers: are non-superimposable mirror images of each other. They occur when a single carbon is bonded to four different types of atoms ...
... The difference between cis- and trans- isomers is very important, especially in metabolic processes. Note: triple bonds do not form diastereomers Enantiomers Enantiomers: are non-superimposable mirror images of each other. They occur when a single carbon is bonded to four different types of atoms ...
Compounds Containing a C=O (Carbonyl) Group
... Triacylglycerols contain three ester groups, each having a long carbon chain (abbreviated as R, R', and R") bonded to the carbonyl group. Triacylglycerols are lipids; that is, they are water-insoluble organic compounds found in biological systems. Animal fats and vegetable oils are composed of triac ...
... Triacylglycerols contain three ester groups, each having a long carbon chain (abbreviated as R, R', and R") bonded to the carbonyl group. Triacylglycerols are lipids; that is, they are water-insoluble organic compounds found in biological systems. Animal fats and vegetable oils are composed of triac ...
α-cleavage of alkenes
... Alkanes: α-cleavage linear: peaks at 15, 29, 43, 57, etc. branched: predominate cleavage at branch points M+ often weak Alkenes: distinct M+ α-cleavage, fragments with mass CnH2n-1 and CnH2n (McLafferty) Cycloalkanes: prominent M+ fragments from loss of side groups ...
... Alkanes: α-cleavage linear: peaks at 15, 29, 43, 57, etc. branched: predominate cleavage at branch points M+ often weak Alkenes: distinct M+ α-cleavage, fragments with mass CnH2n-1 and CnH2n (McLafferty) Cycloalkanes: prominent M+ fragments from loss of side groups ...
Organic Chemistry Fifth Edition
... The Baeyer-Villiger Oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts ketones to esters and cyclic ketones to lactones. The Baeyer-Villiger can be carried out with peracids, such as MCBPA, or with hydrogen peroxide and a Lewis acid. ...
... The Baeyer-Villiger Oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts ketones to esters and cyclic ketones to lactones. The Baeyer-Villiger can be carried out with peracids, such as MCBPA, or with hydrogen peroxide and a Lewis acid. ...
Review
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
Chem 30CL-Lecture 12.. - UCLA Chemistry and Biochemistry
... the poor chemoselectivity of many reagents The use of protective groups usually adds two (or more) steps to the reaction sequence This generates additional cost and additional waste This also decreases atom economy (=atoms used that are ...
... the poor chemoselectivity of many reagents The use of protective groups usually adds two (or more) steps to the reaction sequence This generates additional cost and additional waste This also decreases atom economy (=atoms used that are ...
CH 106 - Clackamas Community College
... Explain why the molecules of alcohols, aldehydes, and ketones are polar and how this affects their melting points, boiling points, and solubility in water. Perform laboratory tests for alcohols (primary and secondary) and aldehydes. Use infrared spectra to help identify the following types of ...
... Explain why the molecules of alcohols, aldehydes, and ketones are polar and how this affects their melting points, boiling points, and solubility in water. Perform laboratory tests for alcohols (primary and secondary) and aldehydes. Use infrared spectra to help identify the following types of ...
Answers, Problem Set 12 (full)... “2,4
... (b) A saturated hydrocarbon is one that contains as many H’s per carbon as possible, and so you could say that it is saturated “with H’s”. It contains no double or triple bonds. Once a double bond or triple bond is formed, there will be less H’s because C only forms 4 bonds before its valence shell ...
... (b) A saturated hydrocarbon is one that contains as many H’s per carbon as possible, and so you could say that it is saturated “with H’s”. It contains no double or triple bonds. Once a double bond or triple bond is formed, there will be less H’s because C only forms 4 bonds before its valence shell ...
course outline - Clackamas Community College
... Describe how amino acids are joined by peptide bonds to make a protein molecule and know the kinds of reactions involved in making and breaking these bonds. Define and recognize descriptions of the primary, secondary, tertiary and quaternary structure of a protein. Know the four major classes of ...
... Describe how amino acids are joined by peptide bonds to make a protein molecule and know the kinds of reactions involved in making and breaking these bonds. Define and recognize descriptions of the primary, secondary, tertiary and quaternary structure of a protein. Know the four major classes of ...
ch221 class 5
... The alkanes can be built from the simplest member, methane (CH 4) by formally inserting successive CH2 (methylene) units in C-H bonds. For the first two alkanes after methane - ethane (C2) and propane (C3) – there is only one way that this can be done: ...
... The alkanes can be built from the simplest member, methane (CH 4) by formally inserting successive CH2 (methylene) units in C-H bonds. For the first two alkanes after methane - ethane (C2) and propane (C3) – there is only one way that this can be done: ...
Selective Incorporation of Difluoromethylene
... physical, and biological properties. Accordingly, the incorporation of fluorine atom(s) or fluorine-containing functionalities into organic molecules has received considerable attention. For decades, extensive reports and reviews mainly focused on the fluorination and perfluoroalkylation (especially ...
... physical, and biological properties. Accordingly, the incorporation of fluorine atom(s) or fluorine-containing functionalities into organic molecules has received considerable attention. For decades, extensive reports and reviews mainly focused on the fluorination and perfluoroalkylation (especially ...
Epoxidation and oxidation reactions using 1,4
... Solid-phase organic synthesis has been used as an efficient technique for the synthesis and screening of a large number of organic compounds.1 Over the past years, numerous techniques have been used to study polymeric resins commonly utilized in solid phase synthesis to allow greater understanding o ...
... Solid-phase organic synthesis has been used as an efficient technique for the synthesis and screening of a large number of organic compounds.1 Over the past years, numerous techniques have been used to study polymeric resins commonly utilized in solid phase synthesis to allow greater understanding o ...
Pyrrolidine-2-carboxylic Acid (l
... from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
... from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
Organic Chemistry II: Here We Go Again!
... (NMR). In addition, many of the chapters in this book have a spectroscopy section at the end where we simply cover the essentials concerning the specific compounds that you study in that chapter. Aromatic compounds and their reactions are a big part of any Organic II course. We introduce you to the ...
... (NMR). In addition, many of the chapters in this book have a spectroscopy section at the end where we simply cover the essentials concerning the specific compounds that you study in that chapter. Aromatic compounds and their reactions are a big part of any Organic II course. We introduce you to the ...
(Organic Chemistry II) Pahlavan
... the compounds have been organized into families, or functional groups, according to common features. Functional groups have characteristic properties and they control the reactivity of the molecule as a whole. Two such functional groups are briefly discussed below. Alcohols represent a class of orga ...
... the compounds have been organized into families, or functional groups, according to common features. Functional groups have characteristic properties and they control the reactivity of the molecule as a whole. Two such functional groups are briefly discussed below. Alcohols represent a class of orga ...
CC 2 097-110..7686hdisk chapter .. Page97
... photolyses in neat MeOH and neat CH3CN confirmed the observations made above by UV-Vis spectra; that is, the yield of 6 is considerably lower in neat MeOH ( ≈ 50% less) and not observed at all in neat CH3CN. These observations are consistent with a mechanism of reaction that requires a protic solven ...
... photolyses in neat MeOH and neat CH3CN confirmed the observations made above by UV-Vis spectra; that is, the yield of 6 is considerably lower in neat MeOH ( ≈ 50% less) and not observed at all in neat CH3CN. These observations are consistent with a mechanism of reaction that requires a protic solven ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.
![(C3H7)3NH[CrO3X],(X=F, Cl), Reagents for Oxidation of](http://s1.studyres.com/store/data/015838257_1-b7e4138a4ed1f989d8dc5b682bb74b7a-300x300.png)