* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Presentation8_108
Survey
Document related concepts
Transcript
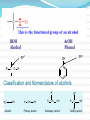
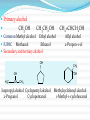
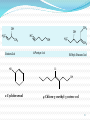
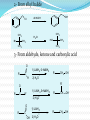
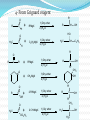
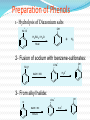
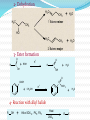
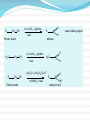
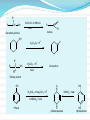
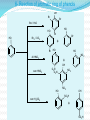
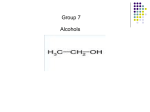
Dr. Shatha Alaqeel ROH Alcohol ArOH Phenol SP3 SP2 OH R C O H Classification and Nomenclature of alcohols R' H3C Carbinol OH R CH2 OH Primary alcohol R CH R'' OH Secondary alcohol R C OH R' Tertiary alcohol Nomenclature 1) Common Nomenclature Alkyl + alcohol CH3CH2OH Ethyl alcohol CH2=CHOH Vinyl alcohol CH2=CHCH2OH Allyl alcohol 2) IUPAC Nomenclature 1) Select the longest continuous carbon chain to which the hydroxyl is directly attached. 2) Change the name of the alkane corresponding to this chain by dropping the final -e and adding the suffix –ol 3) Number the longest continuous carbon chain so as to give the carbon atom bearing the hydroxyl group the lower number. OH H3C Cl OH H3C 4-Ethyl-2-hexanol CH3 H3C OH CH3 5-Chloro-3-methyl-1-heptanol CH3 3-Methyl-1-cyclohexanol Primary alcohol CH3OH CH3CH2OH Common Methyl alcohol Ethyl alcohol IUPAC Methanol Ethanol Secondary and tertiary alcohol OH OH H3C CH2=CHCH2OH Allyl alcohol 2-Propen-1-ol CH3 OH CH CH3 Isopropyl alcohol Cyclopentyl alcohol 2-Propanol Cyclopentanol Methylcyclohexyl alcohol 1-Methyl-1-cyclohexanol 4) OH group is preferred over the double or triple bond in numbering. OH 5-Hepten-2-ol notice the removal of the (e) 5) If a compound contains both OH and a double or triple bond, choose the chain that include them both CH2CH3 even if this is not the longest chain CH3CHC=CH2 OH 3-Ethyl-3-buten-2-ol OH H2C OH HC CH3 3-buten-2-ol HO H3C OH 4-Pentyn-1-ol CH3 CH2 5-Ethyl-5-hexen-3-ol Cl OH 2-Cyclohexenol 4-Chloro-3-methyl-3-octen-1-ol 8 Alcohols containing two hydroxyl groups are commonly called gylcols. In the IUPAC system, the suffix diol is added to the name of the parent hydrocarbon when two hydroxyl groups are present, and the suffix triol is added when there are three OH groups. OH OH OH OH H3C HO OH OH 1,2-Ethanediol 1,2-Propanediol 1,2,3-Propanetriol Ethylene glycol Propylene glycol Glycerol or Glycerin Nomenclature of Phenols 2) Compounds that have a hydroxyl group attached directly to a benzene ring are called phenols OH OH OH OH O2N NO 2 OH NO 2 Cl Cl Cl Cl Br NH2 Phenol 4-Bromo-2-nitrophenol p-Aminophenol Cl NO 2 2,4,6-Trinitrophenol Pentachlorophenol OH OH OH CH3 CH3 o-Cresol m-Cresol p-Cresol HO OH OH CH3 OH OH OH OH Catechol Resorcinol OH HO Hydroquinone Pyrogallol Physical Properties of Alcohols & Phenols Solubility of alcohols The first three members are completely miscible with water. The solubility rapidly decreases with increase in molecular mass. The higher members are almost insoluble in water but are soluble in organic solvents like benzene, ether etc. The solubility of lower alcohols is due to the existence of hydrogen bonds between water and polar -OH group of alcohol molecules. Phenols too are sparingly soluble in water. The -OH group in alcohols and phenols contain a hydrogen bonded to an electronegative oxygen atom. Thus they form hydrogen bonds with water molecules. The solubility increases with branching of chain. Phenols are sparingly soluble in water but readily soluble in organic solvents such as alcohol and ether. •Boiling points of alcohols Boiling point of alcohols are much higher than those of alkenes, halo alkenes or ethers of comparable molecular masses. This is because in alcohols intermolecular hydrogen bonding exists due to which a large amount of energy is required to break these bonds. •Among isomeric alcohols, the boiling point decreases with increase in branching in the alkyl group Boiling points of 1o alcohol < 2o alcohol < 3o alcohol •As number of hydroxyl groups increase the solubility and boiling points increase. Acidity of Alcohols & Phenols Alcohols have weak acidic properties. Phenol is a strong acid than alcohols because the negative charge in oxygen is dispersed by resonance through the benzene ring. Alcohols react with Na (or K) like water to give the alkoxide Introduction of electron-withdrawing groups, such as NO2 or CN, on the ring increases the acidity of phenol, introducing EDG decrease the acidity of phenols OH O 2N NO 2 OH HO NO 2 > > NO 2 OH OH NO 2 > NO 2 > H3C Preparation of alcohols 1- From alkenes H + H2SO 4 H2O OH CH3 CH3 KMnO 4 / OH - OH OH OH 1) RCO 3H 2) H 3O + OH 2- From alkyl halide CH3 Br OH dil KOH Cl CH3 H2O HO CH3 CH3 CH3 CH3 3- From aldehyde, ketone and carboxylic acid O R 1) LiAlH 4 0r NaBH 4 C H 2) H 3O + R R' O R C CH2 OH 1) LiAlH 4 0r NaBH 4 R' 2) H 3O + R CH OH O R 1) LiAlH 4 C OH 2) H 3O + R CH2 OH 4- From Grignard reagent R' O R + C R'MgX H 1) Dry ether R OH 2) H 2O HO O H3C CH + C C2H5MgX H 1) Dry ether 2) H 2O H3C CH C2H5 R' O R C + R' R''MgX 1) Dry ether R 2) H 2O + R'' CH 3MgX 1) Dry ether OH 2) H 2O R'' O + C 2 R''MgX 1) Dry ether R 2) H 2O OR' C + OC 2H5 2 CH 3MgX 1) Dry ether 2) H 2O C OH R'' CH3 O H3C OH CH3 O R C H3C C H3C OH Preparation of Phenols 1- Hydrolysis of Diazonium salts + N2 Cl OH - H2SO4 / H2O + N2 Heat 2- Fusion of sodium with benzene-sulfonates: - O Na SO 3H OH + H3O NaOH / 350 + 3- From alkyl halide: - O Na Cl NaOH / 350 300 atm OH + H3O + Reaction of Alcohols and Phenols 1- Salt Formation + OH 2 R 2 Na OH + + Sodium alkoxide Alcohol as acid H3C - O Na 2R + Na - O Na H3C + Sodium Methoxide Methanol - OH O Na + + Na Sodium Phenoxide H2 2- Dehydration H2C - H3C + CH3 H2O 1 Butene minor CH3 HO H3C + CH3 H2O - 2 Butene major 3- Ester formation O R O + + C H R'OH R + C OH H2O OR' O COOH C + + CH 3OH OCH 3 H + 4- Reaction with alkyl halide R OH + HX or SOX 2 , PX3, PX5 Heat ZnCl 2 R X H2O 5) Oxidation Of Alcohols Alcohols can be oxidised depending on their class For oxidation to take place easily you must have two hydrogen atoms on adjacent C and O atoms. 1° R H H C O + [O] R H 2° R C O + H 2O O + H 2O H H H C O + [O] R R C R This is possible in 1° and 2° alcohols but not in 3° alcohols. 3° R R H C O R + [O] 21 R CH2 OH O Cu or CrO 3 / pyridine heat H adehyde Primary alcohol H3C CH2 OH weak oxidizing reagent R O Cu or CrO 3 / pyridine H3C heat H + H2Cr 2O7 or K 2Cr 2O7/ H R CH2 OH Primary alcohol O R or KMnO 4 / heat OH carboxylic acid R' R CH OH K2Cr 2O 7 or KMnO 4 O R heat R' ketone Secondary alcohol OH + O K2Cr 2O 7 / H R' R + C OH H2CrO 7 / H No reaction heat R'' Tertiary alcohol OH O + HO KMnO 4 / heat H2CrO 7 or Na 2CrO 7 / H or KMnO 4 / heat Phenol O p-Benzoquinone HO Hydroquinone 6- Reaction of aromatic ring of phenols OH Br Br Br2 / H2O HO HO Br HO Br2 / CCl 4 Br + Br HO HO NO 2 dil HNO 3 + OH conc HNO 3 O 2N O 2N HO conc H2SO 4 NO 2 NO 2 OH SO 3H + SO 3H