Hydrogenation of fatty acid methyl ester to fatty alcohol
... Hydrogenation of various types of oleochemicals is a major unit operation in industry. Natural fatty alcohols are important raw materials for surfactants and lubricants and can be produced by catalytic hydrogenation of fatty acid methyl esters (FAME). Commercial multiphase processes are necessarily ...
... Hydrogenation of various types of oleochemicals is a major unit operation in industry. Natural fatty alcohols are important raw materials for surfactants and lubricants and can be produced by catalytic hydrogenation of fatty acid methyl esters (FAME). Commercial multiphase processes are necessarily ...
Carolina Aguirre, Rosa Arrieta, Soledad Anjarí, Andrés Illanes
... conditions hardly compatible with enzyme activity and stability are often required to displace it in favor of synthesis (7,19,20). Yield is expected to increase at reduced water activity since this will depress the competing hydrolytic reactions in the case of kinetically controlled synthesis, as we ...
... conditions hardly compatible with enzyme activity and stability are often required to displace it in favor of synthesis (7,19,20). Yield is expected to increase at reduced water activity since this will depress the competing hydrolytic reactions in the case of kinetically controlled synthesis, as we ...
The Hydroxylation of Aromatic Nitro Compounds by Alkalies
... reacting 8urface. Chemical purity of the potassium hydroxide had no observable effect, but the presence of more than two percent ot water in It lowered the yield markedly. Intensive drying was therefore tried, but neither precautions taken In grinding, nor preliminary fusion of the alkali, nor the a ...
... reacting 8urface. Chemical purity of the potassium hydroxide had no observable effect, but the presence of more than two percent ot water in It lowered the yield markedly. Intensive drying was therefore tried, but neither precautions taken In grinding, nor preliminary fusion of the alkali, nor the a ...
Slide 1
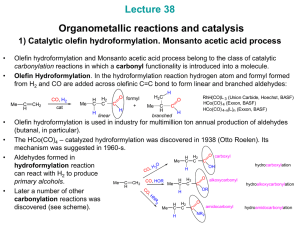
... decomposition. becomes stronger Bulkier PR3 favor greater linear / branched becomes more hydride-like H aldehyde ratios ( up to 8 : 1). CO Finally, more hydride-like Co-H promotes aldehyde OC Co hydrogenation to alcohols: CO ...
... decomposition. becomes stronger Bulkier PR3 favor greater linear / branched becomes more hydride-like H aldehyde ratios ( up to 8 : 1). CO Finally, more hydride-like Co-H promotes aldehyde OC Co hydrogenation to alcohols: CO ...
LDA preparation and other lab techniques
... wet flask). Drying takes longer than for magnesium sulfate. Sit it over the drying agent ~15 minutes for dichloromethane, ~30 minutes for ethyl acetate. It does not work well for ether. Celite: Sometimes it is useful to filter small scale reactions through Celite to remove trace water. ...
... wet flask). Drying takes longer than for magnesium sulfate. Sit it over the drying agent ~15 minutes for dichloromethane, ~30 minutes for ethyl acetate. It does not work well for ether. Celite: Sometimes it is useful to filter small scale reactions through Celite to remove trace water. ...
Identification of Ketones and Aldehydes
... conditions, in this type of reaction a nucleophile (a species that can donate a pair of electrons, in other words a Lewis base) donates a pair of electrons toward the carbonyl carbon forming a single bond to it. At the same time the double bond between the carbonyl carbon and oxygen becomes a single ...
... conditions, in this type of reaction a nucleophile (a species that can donate a pair of electrons, in other words a Lewis base) donates a pair of electrons toward the carbonyl carbon forming a single bond to it. At the same time the double bond between the carbonyl carbon and oxygen becomes a single ...
AMINES
... Ans) This is because FeCl2 formed gets hydrolysed to release HCl in the reaction. Thus, only a small amount of HCl is required to initiate the reaction. Q2) Ammonlysis of R-X is not a preferred method for preparing amines Ans) This method yields a mixture of primary, secondary, tertiary a mines and ...
... Ans) This is because FeCl2 formed gets hydrolysed to release HCl in the reaction. Thus, only a small amount of HCl is required to initiate the reaction. Q2) Ammonlysis of R-X is not a preferred method for preparing amines Ans) This method yields a mixture of primary, secondary, tertiary a mines and ...
Synthesis of p-hydroxy alkyl benzoates
... Parabens [Fig.2.1, 2.2, 2.3 and 2.4] have been attracting great interest because of their importance in synthetic organic chemistry. Parabens have been widely used as antimicrobial preservative agents in foods, beverages, drugs and cosmetics for more than fifty years due to their broad antimicrobial ...
... Parabens [Fig.2.1, 2.2, 2.3 and 2.4] have been attracting great interest because of their importance in synthetic organic chemistry. Parabens have been widely used as antimicrobial preservative agents in foods, beverages, drugs and cosmetics for more than fifty years due to their broad antimicrobial ...
Discovery and synthetic applications of novel silicon
... bottom: silicon-containing unsaturated compounds are so reactive that they can only be isolated through stabilization by introduction of bulky kinetically-protecting groups.5) Obviously, most of these three features (2), (3) and (4) related to silicon-containing molecules are based on the feature (1 ...
... bottom: silicon-containing unsaturated compounds are so reactive that they can only be isolated through stabilization by introduction of bulky kinetically-protecting groups.5) Obviously, most of these three features (2), (3) and (4) related to silicon-containing molecules are based on the feature (1 ...
32.6
... Physical Properties of Halogeno-compounds Preparation of Halogeno-compounds Reactions of Halogeno-compounds Nucleophilic Substitution Reactions ...
... Physical Properties of Halogeno-compounds Preparation of Halogeno-compounds Reactions of Halogeno-compounds Nucleophilic Substitution Reactions ...
Organic chemistry
... There are a number of rules for naming hydrocarbons. • Rule 1: Determine the longest chain of carbon atoms. • Rule 2: Determine which end is nearest to a branch, a double bond or a triple bond. (A double or triple bond takes precedence over a branch if they are equidistant from either end of the c ...
... There are a number of rules for naming hydrocarbons. • Rule 1: Determine the longest chain of carbon atoms. • Rule 2: Determine which end is nearest to a branch, a double bond or a triple bond. (A double or triple bond takes precedence over a branch if they are equidistant from either end of the c ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
chemical properties of sugar
... oxidizing aldehydes but not alcohols such as the Tollen’s test) in basic aqueous solution. The characteristic property of reducing sugars is that, in aqueous medium, they generate one or more compounds containing an aldehyde group. eg. 1: α-D-glucose, which contains a haematical group and, therefo ...
... oxidizing aldehydes but not alcohols such as the Tollen’s test) in basic aqueous solution. The characteristic property of reducing sugars is that, in aqueous medium, they generate one or more compounds containing an aldehyde group. eg. 1: α-D-glucose, which contains a haematical group and, therefo ...
chemical properties of sugar
... oxidizing aldehydes but not alcohols such as the Tollen’s test) in basic aqueous solution. The characteristic property of reducing sugars is that, in aqueous medium, they generate one or more compounds containing an aldehyde group. eg. 1: α-D-glucose, which contains a haematical group and, therefo ...
... oxidizing aldehydes but not alcohols such as the Tollen’s test) in basic aqueous solution. The characteristic property of reducing sugars is that, in aqueous medium, they generate one or more compounds containing an aldehyde group. eg. 1: α-D-glucose, which contains a haematical group and, therefo ...
Slides from Chapters 1,2
... for (HCONH)!. The true structure is a composite of both resonance forms and is called a resonance hybrid. • The hybrid shows characteristics of both structures. • Resonance allows certain electron pairs to be delocalized over several atoms, and this delocalization adds stability. • A molecule wit ...
... for (HCONH)!. The true structure is a composite of both resonance forms and is called a resonance hybrid. • The hybrid shows characteristics of both structures. • Resonance allows certain electron pairs to be delocalized over several atoms, and this delocalization adds stability. • A molecule wit ...
Micellar Catalytic Effect of Cetyltrimethylammonium Bromide
... presence of potassium carbonate. However, problems arise when the O-allylation reaction with eugenol. This reaction is ether compounds formation which requires heat conditions but in eugenol it will followed by Claisen rearrangement reaction. Because of that the use of heat on allylation of eugenol ...
... presence of potassium carbonate. However, problems arise when the O-allylation reaction with eugenol. This reaction is ether compounds formation which requires heat conditions but in eugenol it will followed by Claisen rearrangement reaction. Because of that the use of heat on allylation of eugenol ...
Lewis base-assisted Lewis acid-catalyzed selective
... was probed, and the results are shown in Table 2. First, the α‐phenylethanols bearing para‐substituted groups were stud‐ ied (entries 1–6). It was found that halide, alkyl and aryl group substituted alcohols readily yielded their corresponding ole‐ fins. The presence of an el ...
... was probed, and the results are shown in Table 2. First, the α‐phenylethanols bearing para‐substituted groups were stud‐ ied (entries 1–6). It was found that halide, alkyl and aryl group substituted alcohols readily yielded their corresponding ole‐ fins. The presence of an el ...
Rutgers...Ch17 Reactions of Aromatic Compounds
... This is followed by loss of a proton, giving tbutyl benzene as the product. ...
... This is followed by loss of a proton, giving tbutyl benzene as the product. ...
Benzyne Mechanism
... Side-Chain Halogenation Benzylic position is the most reactive. Chlorination is not as selective as bromination, results in mixtures. Br2 reacts only at the benzylic position. Br CH2CH2CH3 ...
... Side-Chain Halogenation Benzylic position is the most reactive. Chlorination is not as selective as bromination, results in mixtures. Br2 reacts only at the benzylic position. Br CH2CH2CH3 ...
CH 2
... • -OH group • Higher than expected boiling point (based on molecular weight) • Naming is based on longest carbon chain attached to –OH group – End in -ol ...
... • -OH group • Higher than expected boiling point (based on molecular weight) • Naming is based on longest carbon chain attached to –OH group – End in -ol ...
Chapter 13
... class) an alcohol and an ether given a structural formula. 2. Be able to draw an alcohol and an ether given the name of a compound (given using IUPAC nomenclature rules or common names if given in class). 3. Be able to list six physical properties of alcohols. 4. Be able to list six physical propert ...
... class) an alcohol and an ether given a structural formula. 2. Be able to draw an alcohol and an ether given the name of a compound (given using IUPAC nomenclature rules or common names if given in class). 3. Be able to list six physical properties of alcohols. 4. Be able to list six physical propert ...
Making esters from carboxylic acids and alcohols
... Esters are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. We shall just be looking at cases where it is replaced by an alkyl group, but it could equally well be an aryl group (one ...
... Esters are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. We shall just be looking at cases where it is replaced by an alkyl group, but it could equally well be an aryl group (one ...
01. Introduction of bioorganic chemistry. Classification, structure
... So far as alkenes have double bonds they are more reactive than alkanes and undergo addition reactions. Some of the reagents that can be added to alkenes: hydrogen, halogens, hydrogen halides, water, sulfuric acid, etc. ...
... So far as alkenes have double bonds they are more reactive than alkanes and undergo addition reactions. Some of the reagents that can be added to alkenes: hydrogen, halogens, hydrogen halides, water, sulfuric acid, etc. ...
IN VITRO Research Article S. NITHIYA *, N. KARTHIK
... used method to evaluate antioxidant activities in a relatively short time compared with other methods. The effect of antioxidants on DPPH radical scavenging was thought to be due to their electron/hydrogen donating ability14. DPPH is a stable free radical and ...
... used method to evaluate antioxidant activities in a relatively short time compared with other methods. The effect of antioxidants on DPPH radical scavenging was thought to be due to their electron/hydrogen donating ability14. DPPH is a stable free radical and ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.