ETHER
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
... Has a pair of alkyl or atomic groups attached to a linking oxygen atom. Functional group is ROR Have primary, secondary, and tertiary structures ...
1.7 FUNCTIONAL GROUPS
... Certain combinations of bonds show up repeatedly in organic chemistry and organic chemists give those bonding combinations specific names. It is very useful to know the names of those specific types of bonds. Examples are shown below and you should make flash cards and learn them by heart. There can ...
... Certain combinations of bonds show up repeatedly in organic chemistry and organic chemists give those bonding combinations specific names. It is very useful to know the names of those specific types of bonds. Examples are shown below and you should make flash cards and learn them by heart. There can ...
Chapter 20 Amines - FIU Faculty Websites
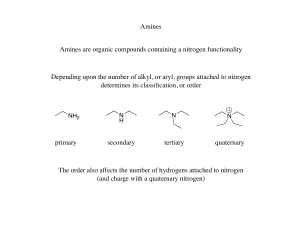
... • 10 Amines are named using either systematic or common names. • To assign a systematic name, find the longest continuous chain bonded to the amine nitrogen, and change the –e ending of the parent alkane to the suffix –amine. Then use the usual rules of nomenclature to number the chain and name the ...
... • 10 Amines are named using either systematic or common names. • To assign a systematic name, find the longest continuous chain bonded to the amine nitrogen, and change the –e ending of the parent alkane to the suffix –amine. Then use the usual rules of nomenclature to number the chain and name the ...
Microsoft Word
... We found that La(NO3)36H2O is an efficient and mild acidic catalyst for the acetylation of alcohols with Ac2O under solvent-free conditions. In order to establish the catalytic activity of La(NO3)36H2O, we carried out the acetylation of glucose diacetonide (1 mmol) with acetic anhydride (1.2 mmol) u ...
... We found that La(NO3)36H2O is an efficient and mild acidic catalyst for the acetylation of alcohols with Ac2O under solvent-free conditions. In order to establish the catalytic activity of La(NO3)36H2O, we carried out the acetylation of glucose diacetonide (1 mmol) with acetic anhydride (1.2 mmol) u ...
Chapter 19
... The amide does not generally react further because the lone pair of electrons is delocalized ...
... The amide does not generally react further because the lone pair of electrons is delocalized ...
18.10 CONJUGATE ADDITIONS
... adds in the first step. This reaction does not occur unless there is a group attached to the double bond that can help stabilize, by resonance, the carbanion intermediate. In many cases this is the carbonyl group of an aldehyde or a ketone. However, other groups, such as the carbonyl group of an est ...
... adds in the first step. This reaction does not occur unless there is a group attached to the double bond that can help stabilize, by resonance, the carbanion intermediate. In many cases this is the carbonyl group of an aldehyde or a ketone. However, other groups, such as the carbonyl group of an est ...
Organic chemistry - Delivery guide
... p-orbitals above and below the bonding C atoms) and a σ-bond (overlap of orbitals directly between the bonding atoms) (see also 4.1.2 a); restricted rotation of the π-bond (b) explanation of the trigonal planar shape and bond angle around each carbon in the C=C of alkenes in terms of electron pair ...
... p-orbitals above and below the bonding C atoms) and a σ-bond (overlap of orbitals directly between the bonding atoms) (see also 4.1.2 a); restricted rotation of the π-bond (b) explanation of the trigonal planar shape and bond angle around each carbon in the C=C of alkenes in terms of electron pair ...
Practice Problem - HCC Southeast Commons
... – The cationic intermediate was first proposed by G. W. Wheland and is often called the Wheland intermediate ...
... – The cationic intermediate was first proposed by G. W. Wheland and is often called the Wheland intermediate ...
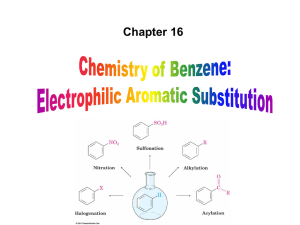
Aromatic Compounds
... Alkyl groups have an electron-donating inductive effect Nitration of toluene occurs ortho and para to the alkyl group because a resonance form places the positive charge directly on the alkyl-substituted carbon where it can be stabilized by the electron-donating inductive effect of the alkyl group ...
... Alkyl groups have an electron-donating inductive effect Nitration of toluene occurs ortho and para to the alkyl group because a resonance form places the positive charge directly on the alkyl-substituted carbon where it can be stabilized by the electron-donating inductive effect of the alkyl group ...
alcohols ws 1 - Chesterhouse School
... (d) Lactic acid is chiral. Draw displayed formulae of the two optical isomers of lactic acid clearly showing their three-dimensional structures. Indicate with an asterisk (*) the chiral carbon atom in each. ...
... (d) Lactic acid is chiral. Draw displayed formulae of the two optical isomers of lactic acid clearly showing their three-dimensional structures. Indicate with an asterisk (*) the chiral carbon atom in each. ...
102 Lecture Ch19
... Preparation of Amides from Carboxylic Acids • Amines (except tertiary) can react with carboxylic acids to produce amides (amidation) • The reaction is similar to esterification • Unfortunately, the reaction is of little synthetic use - amines are bases and will remove a proton from a carboxylic aci ...
... Preparation of Amides from Carboxylic Acids • Amines (except tertiary) can react with carboxylic acids to produce amides (amidation) • The reaction is similar to esterification • Unfortunately, the reaction is of little synthetic use - amines are bases and will remove a proton from a carboxylic aci ...
Organic Chemistry II Laboratory
... product. The two bromines which add to the double bond will be on opposite sides of the plane of the former pibond (in this case, the plane of the cyclopentane ring). Since the bromide ion could attack either one of the two carbons of the cyclic bromoniun ion intermediate, two different products are ...
... product. The two bromines which add to the double bond will be on opposite sides of the plane of the former pibond (in this case, the plane of the cyclopentane ring). Since the bromide ion could attack either one of the two carbons of the cyclic bromoniun ion intermediate, two different products are ...
print
... Achiral Reactions • Achiral starting materials react with achiral reagents to give either achiral products, or a racemic mixture of two enantiomers. ...
... Achiral Reactions • Achiral starting materials react with achiral reagents to give either achiral products, or a racemic mixture of two enantiomers. ...
Oxidising Alcohols
... All of the products of oxidation of alcohols contain a C=O group. The C=O group is known as a carbonyl group. ...
... All of the products of oxidation of alcohols contain a C=O group. The C=O group is known as a carbonyl group. ...
naming and isomerism
... four different groups attached is called a chiral (asymmetric) carbon atom ...
... four different groups attached is called a chiral (asymmetric) carbon atom ...
Chemistry Unit 1
... Alkanes are saturated hydrocarbons. They contain chains of carbon atoms linked by single bonds only. Every carbon atom in the molecule forms four single covalent bonds with other atoms. Alkanes have the general formula CnH2n+2, where, n = 1, 2, 3 . . . Using this general formula, we can write the mo ...
... Alkanes are saturated hydrocarbons. They contain chains of carbon atoms linked by single bonds only. Every carbon atom in the molecule forms four single covalent bonds with other atoms. Alkanes have the general formula CnH2n+2, where, n = 1, 2, 3 . . . Using this general formula, we can write the mo ...
Synthesis and Structure of Alcohols
... Acetylides and carbonyl compounds Organometallic Reagents for Alcohol Synthesis When a compound has a covalent bond between a carbon and a metal, it is called an organometallic compound. The two most common types of organometallic are Grignard reagents and organolithium reagents (although there are ...
... Acetylides and carbonyl compounds Organometallic Reagents for Alcohol Synthesis When a compound has a covalent bond between a carbon and a metal, it is called an organometallic compound. The two most common types of organometallic are Grignard reagents and organolithium reagents (although there are ...
Synthesis_of_Organometallic_Compounds
... • less application in organic synthesis than palladium compounds, probably because their chemistry is more complicated. ...
... • less application in organic synthesis than palladium compounds, probably because their chemistry is more complicated. ...
Carboxylic Acids, Amines, and Amides
... -Amines smell like rotten fish. • Many amines are physiologically active. -Smaller amines are irritating to the skin, eyes, and mucous membrane and are toxic by ingestion. ...
... -Amines smell like rotten fish. • Many amines are physiologically active. -Smaller amines are irritating to the skin, eyes, and mucous membrane and are toxic by ingestion. ...
6.10 Acid-Catalyzed Hydration of Alkenes
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
Organic Synthesis - National Open University of Nigeria
... acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most reactions are effected with m-chloroperbenzoic acid. This is more stable than the other acids, which usually have to be prepared immediately before use, and is commercially available. The reaction occurs under mild ...
... acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most reactions are effected with m-chloroperbenzoic acid. This is more stable than the other acids, which usually have to be prepared immediately before use, and is commercially available. The reaction occurs under mild ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.