Scheme A Topic Checklist Atomic Structure 1.1
... understand that E-Z isomers exist due to restricted rotation about the C=C bond understand that the double bond in an alkene is a centre of high electron density Addition reactions of alkenes understand the mechanism of electrophilic addition of alkenes with HBr, H2SO4 and Br2 know that bromine ca ...
... understand that E-Z isomers exist due to restricted rotation about the C=C bond understand that the double bond in an alkene is a centre of high electron density Addition reactions of alkenes understand the mechanism of electrophilic addition of alkenes with HBr, H2SO4 and Br2 know that bromine ca ...
1. 4-methyl-4-octanol oxidizes to form a) 4-methyl-4
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
File - Grade 12 Chemistry
... Smaller than five carbons, very reactive. Rings of carbon atoms. Isomers ...
... Smaller than five carbons, very reactive. Rings of carbon atoms. Isomers ...
PRACTICE – Naming and Writing Ionic Compounds
... Na2S2O3(aq) + 4Cl2(g) + 5H2O(aq) 2NaHSO4(aq) + 8HCl(aq) a. How many moles of Na2S2O3 are needed to react with 0.12mol of Cl2? ...
... Na2S2O3(aq) + 4Cl2(g) + 5H2O(aq) 2NaHSO4(aq) + 8HCl(aq) a. How many moles of Na2S2O3 are needed to react with 0.12mol of Cl2? ...
Lecture 16 Aromatic Diazonium Salts
... Azo-compounds that contain both an acidic and a basic group can be utilized as indicators since the colours of the conjugate acid and the conjugate base are different. Examples are methyl orange and methyl red which are prepared by coupling of dimethylaniline with diazotized sulfanilic acid and diaz ...
... Azo-compounds that contain both an acidic and a basic group can be utilized as indicators since the colours of the conjugate acid and the conjugate base are different. Examples are methyl orange and methyl red which are prepared by coupling of dimethylaniline with diazotized sulfanilic acid and diaz ...
Lex 4.1 Alcohols Thiols Ethers
... • In order to be a polar molecule it needs to have both: a. Polar bonds and b. Polar symmetry Polar Symmetries: Net Dipole Dipole Bent Pyrimidal δδ+ δδ+ ...
... • In order to be a polar molecule it needs to have both: a. Polar bonds and b. Polar symmetry Polar Symmetries: Net Dipole Dipole Bent Pyrimidal δδ+ δδ+ ...
Alkene-Addn-PartB-2012-ques
... transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon ...
... transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon ...
Organic Nomenclature
... Organic compounds are compounds containing carbon bonded to other nonmetals such as hydrogen, nitrogen, oxygen, or the halogens. The term organic comes from the old idea that all carbon containing compounds had to be produced by a living organism. While living organisms produce a vast number of orga ...
... Organic compounds are compounds containing carbon bonded to other nonmetals such as hydrogen, nitrogen, oxygen, or the halogens. The term organic comes from the old idea that all carbon containing compounds had to be produced by a living organism. While living organisms produce a vast number of orga ...
Lecture 3 Polar and non-polar covalent bonds
... The three major types of bonding found in most compounds are ionic, polar covalent, and nonpolar covalent bonds. • An ionic bond is the transfer of electrons. • A covalent bond is the sharing of electrons. • A polar covalent bond is between atoms of different electronegativity. • A nonpolar covalent ...
... The three major types of bonding found in most compounds are ionic, polar covalent, and nonpolar covalent bonds. • An ionic bond is the transfer of electrons. • A covalent bond is the sharing of electrons. • A polar covalent bond is between atoms of different electronegativity. • A nonpolar covalent ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
... The parent chain is numbered to give the ketone carbonyl the lowest possible number In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl ...
Survival Organic Chemistry Molecular Models The goal in this
... understanding of the spatial relationships of atoms in molecules. What you need to remember for this experience: Rule of eight, valence electrons, simple electron (Lewis) structures, covalent bond, ionic bond, single, double and triple bonds, and bond angles. As a way to get a handle on these concep ...
... understanding of the spatial relationships of atoms in molecules. What you need to remember for this experience: Rule of eight, valence electrons, simple electron (Lewis) structures, covalent bond, ionic bond, single, double and triple bonds, and bond angles. As a way to get a handle on these concep ...
Kekulé structure of benzene
... There are many different types of isomers, including those in which the molecules have different functional groups. Different functional groups different classes, different reactivities. E.g. C2H6O describes both an alcohol and ether ...
... There are many different types of isomers, including those in which the molecules have different functional groups. Different functional groups different classes, different reactivities. E.g. C2H6O describes both an alcohol and ether ...
functional group review
... • When phenolic drugs needed to dissolve in aq-environment then they are treated with aq-bases to form salt. See next slide ...
... • When phenolic drugs needed to dissolve in aq-environment then they are treated with aq-bases to form salt. See next slide ...
Chapter 17 Aldehydes and Ketones
... The carbonyl group of an aldehyde or ketone is reduced to an -CHOH group by hydrogen in the presence of a transition-metal catalyst. • Reduction of an aldehyde gives a primary alcohol. • Reduction a ketone gives a secondary alcohol. ...
... The carbonyl group of an aldehyde or ketone is reduced to an -CHOH group by hydrogen in the presence of a transition-metal catalyst. • Reduction of an aldehyde gives a primary alcohol. • Reduction a ketone gives a secondary alcohol. ...
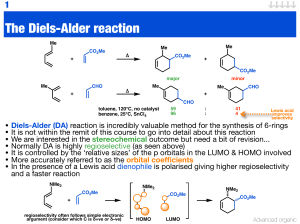
The Diels-Alder reaction
... • Another hetero-Diels-Alder reaction • It looks very similar to the previous reaction but... • It is believed that only one hydrogen bond activates the aldehyde • The other is used to form a rigid chiral environment for the reaction Advanced organic ...
... • Another hetero-Diels-Alder reaction • It looks very similar to the previous reaction but... • It is believed that only one hydrogen bond activates the aldehyde • The other is used to form a rigid chiral environment for the reaction Advanced organic ...
Ch 20 Carboxylic Acids and Nitriles
... they have higher melting points and boiling points as well. - The H-bonding properties also make the smaller acid molecules soluble in water. ...
... they have higher melting points and boiling points as well. - The H-bonding properties also make the smaller acid molecules soluble in water. ...
Alcohols, Phenols, Thiols, and Ethers
... • A number giving the position of each of the hydroxyl groups is needed in these cases. ...
... • A number giving the position of each of the hydroxyl groups is needed in these cases. ...
carbon compounds - Badhan Education
... Alkane. They are saturated hydrocarbons with general formula CnH2n + 2 where ‘n’ is equal to 1, 2, 3, 4, 5, etc. e. g., CH4 (methane), C2H6 (ethane), C3H8 (propane). Paraffins. It means little affinity or reactivity. Alkanes are also called paraffins because they have little affinity or reactivity. ...
... Alkane. They are saturated hydrocarbons with general formula CnH2n + 2 where ‘n’ is equal to 1, 2, 3, 4, 5, etc. e. g., CH4 (methane), C2H6 (ethane), C3H8 (propane). Paraffins. It means little affinity or reactivity. Alkanes are also called paraffins because they have little affinity or reactivity. ...
carboxylic acids
... • Carboxylic acids have significantly higher boiling points than their corresponding alcohols or alkanes. ...
... • Carboxylic acids have significantly higher boiling points than their corresponding alcohols or alkanes. ...
3 Alkanes with Substituents GOB Structures
... 12.3 Alkanes with Substituents When an alkane has four or more carbon atoms, the atoms can be arranged so that a side group called a branch or substituent is attached to a carbon chain. ...
... 12.3 Alkanes with Substituents When an alkane has four or more carbon atoms, the atoms can be arranged so that a side group called a branch or substituent is attached to a carbon chain. ...
Carbon Bond - Rutgers Chemistry
... the structures of important pharmaceuticals, the most extensively studied have been alkenylated benzimidazoles. These compounds also undergo cyclization with satisfying generality. In addition to exploring the scope of the cyclizations, we have also investigated their mechanism.23 Through the use of ...
... the structures of important pharmaceuticals, the most extensively studied have been alkenylated benzimidazoles. These compounds also undergo cyclization with satisfying generality. In addition to exploring the scope of the cyclizations, we have also investigated their mechanism.23 Through the use of ...
An Overview of Carbonyl Compound Chemistry
... as the final products. For example, when organometallic compounds, Grignard or organolithium reagents, are used as the carbanion species, they can react with the ketone or aldehyde intermediates very rapidly to form a second tetrahedral intermediate, in which no good leaving groups are present. Over ...
... as the final products. For example, when organometallic compounds, Grignard or organolithium reagents, are used as the carbanion species, they can react with the ketone or aldehyde intermediates very rapidly to form a second tetrahedral intermediate, in which no good leaving groups are present. Over ...
Chapter 21 aldehydes and ketones
... • Like gem-diol formation, the synthesis of acetals is reversible, and often, the equilibrium favors the reactants. • In acetal synthesis, since water is formed as a by-product, the equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. • Drivi ...
... • Like gem-diol formation, the synthesis of acetals is reversible, and often, the equilibrium favors the reactants. • In acetal synthesis, since water is formed as a by-product, the equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. • Drivi ...
Hydroxyl Compounds
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
chapter 6-hydroxyl compounds
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
... - Reason: a more highly substituted alkyl group inhibits solvation of the alkoxide ion and drives the dissociation equilibrium to the left. - For example: methanol is more acidic than t-butyl alcohol. • The present of electron-withdrawing atoms enhances the acidity of alcohols. - Reason: the electro ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.