physicochemical properties of organic medicinal agents
... susceptible to electrophilic attack than alkenes; however, this is not the case. Alkynes are less susceptible to attack by electrophiles than alkenes. This is thought to be due to how tightly the pi electrons are held by alkynes and the fact that a relatively unstable vinyl carbocation would be form ...
... susceptible to electrophilic attack than alkenes; however, this is not the case. Alkynes are less susceptible to attack by electrophiles than alkenes. This is thought to be due to how tightly the pi electrons are held by alkynes and the fact that a relatively unstable vinyl carbocation would be form ...
3.10 aromatic chemistry
... Naming aromatic compounds where another functional group takes priority: ...
... Naming aromatic compounds where another functional group takes priority: ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to
... equilibrium between the carbonyl compound and its hydrate l The hydrate is also called a gem-diol l The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is stericallycrowded l Aqueous solution of formaldehyde is largely in hydrated form ...
... equilibrium between the carbonyl compound and its hydrate l The hydrate is also called a gem-diol l The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is stericallycrowded l Aqueous solution of formaldehyde is largely in hydrated form ...
14_06_10.html
... Synthesis of Alcohols Using Organolithium Reagents Organolithium reagents react with aldehydes and ketones in the same way that Grignard reagents do. ...
... Synthesis of Alcohols Using Organolithium Reagents Organolithium reagents react with aldehydes and ketones in the same way that Grignard reagents do. ...
Yeast Reduction #812
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the –OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the –OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
Mock-UP - GEOCITIES.ws
... (RCH2OH) the stronger acid than secondary alcohols (RRCHOH), these are stronger acid than tertiary alcohols (RRRCOH), because of for return to the group alkyl related to carbon atom, that related to (OH) group, whenever electrons density to increase on the oxygen atom, then to find difficulty to ion ...
... (RCH2OH) the stronger acid than secondary alcohols (RRCHOH), these are stronger acid than tertiary alcohols (RRRCOH), because of for return to the group alkyl related to carbon atom, that related to (OH) group, whenever electrons density to increase on the oxygen atom, then to find difficulty to ion ...
Barton Deoxygenation
... Hydrogenation of Alkynes Hydrogenation: anti addition – Synthesis of trans-alkenes A dissolving metal reaction which uses lithium or sodium metal in low temperature ammonia or amine solvent produces trans-alkenes. This dissolving metal reduction process is different than other catalytic hydrogenati ...
... Hydrogenation of Alkynes Hydrogenation: anti addition – Synthesis of trans-alkenes A dissolving metal reaction which uses lithium or sodium metal in low temperature ammonia or amine solvent produces trans-alkenes. This dissolving metal reduction process is different than other catalytic hydrogenati ...
alcohol
... In the body, similar oxidation are accomplished by enzymes, together with rather complex coenzyme called nicotinamide adenine dinucleotide, NAD+. This reaction takes place in the liver and is a key step in the body’s attempt to rid itself of imbibed alcohol. The resulting acetaldehyde is also toxic ...
... In the body, similar oxidation are accomplished by enzymes, together with rather complex coenzyme called nicotinamide adenine dinucleotide, NAD+. This reaction takes place in the liver and is a key step in the body’s attempt to rid itself of imbibed alcohol. The resulting acetaldehyde is also toxic ...
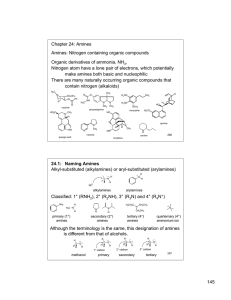
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
Alcohols, Phenols and Ethers
... •-diols or –triols mean two or three hydroxy groups in the molecule •Number the hydroxy group carbons with the lowest possible numbers •Prefix uses the full parent name, i.e butane ...
... •-diols or –triols mean two or three hydroxy groups in the molecule •Number the hydroxy group carbons with the lowest possible numbers •Prefix uses the full parent name, i.e butane ...
Chem 342 Jasperse Syllabus 1 Organic Chemistry II READING
... The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end up never getting this material in either semester. Topics center around the ...
... The following are reading sections and problems associated with one chapter that was covered at MSUM in Organic I but is covered at NDSU in Organic II. Thus, if you are an NDSU student taking Organic II at MSUM, you will end up never getting this material in either semester. Topics center around the ...
Zn mediated regioselective Barbier reaction of propargylic bromides
... high selectivity [9]. It was reported recently that allenic alcohols were obtained with high selectivity by indium-mediated coupling of propargylic halides with aldehydes in aqueous media [7]. The zinc mediated regioselective synthesis of allenic alcohols in media containing water is not documented. ...
... high selectivity [9]. It was reported recently that allenic alcohols were obtained with high selectivity by indium-mediated coupling of propargylic halides with aldehydes in aqueous media [7]. The zinc mediated regioselective synthesis of allenic alcohols in media containing water is not documented. ...
Experiment 7-Reduction
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
Learning Guide for Chapter 16
... What do thioethers often smell like? oysters that have been left in the fridge too long What thioether did we use in the reaction of alkenes with ozone? ...
... What do thioethers often smell like? oysters that have been left in the fridge too long What thioether did we use in the reaction of alkenes with ozone? ...
Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under
... using other Michael acceptors with various aromatic and aliphatic amines. As shown in Table 1, the Michael addition of various aliphatic amines and aryl amines carrying either electron-donating or electron-withdrawing groups were successfully reacted with PTEA or PEEA to produce their corresponding ...
... using other Michael acceptors with various aromatic and aliphatic amines. As shown in Table 1, the Michael addition of various aliphatic amines and aryl amines carrying either electron-donating or electron-withdrawing groups were successfully reacted with PTEA or PEEA to produce their corresponding ...
Study Guide for Exam 4 Chapter 17
... From their structural or line-angle formulas, write IUPAC names of carboxylic acids, carboxylic salts, anhydrides, esters, and amides From their names, draw condensed structural or line-angle formulas of carboxylic acids, carboxylic salts, anhydrides, esters, and amides. Describe the physical ...
... From their structural or line-angle formulas, write IUPAC names of carboxylic acids, carboxylic salts, anhydrides, esters, and amides From their names, draw condensed structural or line-angle formulas of carboxylic acids, carboxylic salts, anhydrides, esters, and amides. Describe the physical ...
Chapter 11 Carboxylic Anhydrides, Esters, and Amides
... The functional group of an ester is a carbonyl group bonded to an -OR group. R may be alkyl or aryl. • Both IUPAC and common names of esters are derived from the names of the parent carboxylic acids. • Name the alkyl or aryl group bonded to oxygen first, followed by the name of the acid; replace the ...
... The functional group of an ester is a carbonyl group bonded to an -OR group. R may be alkyl or aryl. • Both IUPAC and common names of esters are derived from the names of the parent carboxylic acids. • Name the alkyl or aryl group bonded to oxygen first, followed by the name of the acid; replace the ...
Crown Ethers
... alcohols. • Ether molecules can hydrogen bond with water and alcohol molecules. • They are hydrogen bond acceptors. © 2013 Pearson Education, Inc. ...
... alcohols. • Ether molecules can hydrogen bond with water and alcohol molecules. • They are hydrogen bond acceptors. © 2013 Pearson Education, Inc. ...
T10 SL - MsReenChemistry
... 2-chloro-3-methylbutane reacts with sodium hydroxide via an SN2 mechanism. Explain the mechanism by using curly arrows to represent the movement of electron pairs. ...
... 2-chloro-3-methylbutane reacts with sodium hydroxide via an SN2 mechanism. Explain the mechanism by using curly arrows to represent the movement of electron pairs. ...
Chapter 20: Carboxylic Acids and Nitriles
... • Oxidation of a substituted alkylbenzene with KMnO4 or Na2Cr2O7 gives a substituted benzoic acid (see Section 16.10) • 1° and 2° alkyl groups can be oxidized, but tertiary groups are not ...
... • Oxidation of a substituted alkylbenzene with KMnO4 or Na2Cr2O7 gives a substituted benzoic acid (see Section 16.10) • 1° and 2° alkyl groups can be oxidized, but tertiary groups are not ...
CHM102 - National Open University of Nigeria
... hydrogen atoms to form CH2. But actually it forms four bonds with four hydrogen atoms to give CH4. Pauling proposed that this could be explained by using orbital hybridization. In this method, atomic orbitals are mixed to yield the new hybrid orbitals. In this case, in the first step on of the 2s el ...
... hydrogen atoms to form CH2. But actually it forms four bonds with four hydrogen atoms to give CH4. Pauling proposed that this could be explained by using orbital hybridization. In this method, atomic orbitals are mixed to yield the new hybrid orbitals. In this case, in the first step on of the 2s el ...
enzymatic And Limited Industrial Use
... A stereospecific reaction is one in which a single starting material yields only a single stereoisomer ...
... A stereospecific reaction is one in which a single starting material yields only a single stereoisomer ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.